Farnesoid X Receptor Activation Protects Liver From Ischemia/Reperfusion Injury by Up-Regulating Small Heterodimer Partner in Kupffer Cells
- PMID: 32258949
- PMCID: PMC7109340
- DOI: 10.1002/hep4.1478
Farnesoid X Receptor Activation Protects Liver From Ischemia/Reperfusion Injury by Up-Regulating Small Heterodimer Partner in Kupffer Cells
Abstract
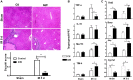
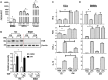
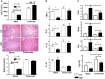
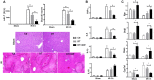
Farnesoid X receptor (FXR) is the nuclear receptor of bile acids and is involved in innate immune regulation. FXR agonists have been shown to protect multiple organs from inflammatory tissue injuries. Because liver expresses high levels of FXR, we explored the potential therapeutic benefits and underlying mechanisms of pharmacologic FXR activation in a murine model of partial liver warm ischemia. Pretreatment of mice with FXR agonist 3-(2,6-dichlorophenyl)-4-(3'-carboxy-2-chlorostilben-4-yl)oxymethyl-5-isopropylisoxazole (GW4064) attenuated liver ischemia/reperfusion injuries (IRIs) in wild-type but not FXR knockout mice. Posttreatment with GW4064 facilitated liver recovery from IRI. Mechanistically, Kupffer cells (KCs) expressed much higher levels of FXR than bone marrow-derived macrophages (BMMs). Pretreatment of KCs but not BMMs with GW4064 resulted in lower tumor necrosis factor α but higher interleukin-10 expressions following toll-like receptor stimulation. FXR-targeted gene small heterodimer partner (SHP) was critical for the regulation of KC response by GW4064. In vivo, the depletion of KCs but not cluster of differentiation (CD) 11b+ cells or knockdown of SHP diminished the immune regulatory effect of GW4064 in liver IRI. Thus, FXR activation protects liver from IRI by up-regulating SHP in KCs to inhibit the liver proinflammatory response.
© 2020 The Authors. Hepatology Communications published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures


References
-
- Ohkohchi N. Mechanisms of preservation and ischemic/reperfusion injury in liver transplantation. Transplant Proc 2002;34:2670‐2673. - PubMed
-
- Tsai YF, Liu FC, Sung WC, Lin CC, Chung PC, Lee WC, et al. Ischemic reperfusion injury‐induced oxidative stress and pro‐inflammatory mediators in liver transplantation recipients. Transplant Proc 2014;46:1082‐1086. - PubMed
-
- Kaczorowski DJ, Tsung A, Billiar TR. Innate immune mechanisms in ischemia/reperfusion. Front Biosci (Elite Ed) 2009;1:91‐98. - PubMed

