Two-stage metabolic remodelling in macrophages in response to lipopolysaccharide and interferon-γ stimulation
- PMID: 32259027
- PMCID: PMC7108803
- DOI: 10.1038/s42255-019-0083-2
Two-stage metabolic remodelling in macrophages in response to lipopolysaccharide and interferon-γ stimulation
Abstract
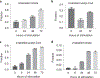
In response to signals associated with infection or tissue damage, macrophages undergo a series of dynamic phenotypic changes. Here we show that during the response to LPS and interferon-γ stimulation, metabolic reprogramming in macrophages is also highly dynamic. Specifically, the TCA cycle undergoes a two-stage remodeling: the early stage is characterized by a transient accumulation of intermediates including succinate and itaconate, while the late stage is marked by the subsidence of these metabolites. The metabolic transition into the late stage is largely driven by the inhibition of pyruvate dehydrogenase complex (PDHC) and oxoglutarate dehydrogenase complex (OGDC), which is controlled by the dynamic changes in lipoylation state of both PDHC and OGDC E2 subunits and phosphorylation of PDHC E1 subunit. This dynamic metabolic reprogramming results in a transient metabolic state that strongly favors HIF-1α stabilization during the early stage, which subsides by the late stage; consistently, HIF-1α levels follow this trend. This study elucidates a dynamic and mechanistic picture of metabolic reprogramming in LPS and interferon-γ stimulated macrophages, and provides insights into how changing metabolism can regulate the functional transitions in macrophages over a course of immune response.
Figures
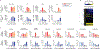
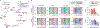
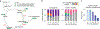
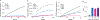
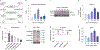


References
-
- Janeway CA & Medzhitov R Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002). - PubMed
-
- Liew FY, Xu D, Brint EK & O’Neill LAJ Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458 (2005). - PubMed
-
- Foster SL, Hargreaves DC & Medzhitov R Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

