Biomaterials for Modulating Lymphatic Function in Immunoengineering
- PMID: 32259064
- PMCID: PMC7088982
- DOI: 10.1021/acsptsci.9b00047
Biomaterials for Modulating Lymphatic Function in Immunoengineering
Abstract
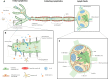
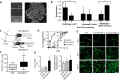
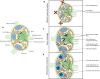
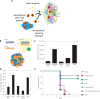
Immunoengineering is a rapidly growing and interdisciplinary field focused on developing tools to study and understand the immune system, then employing that knowledge to modulate immune response for the treatment of disease. Because of its roles in housing a substantial fraction of the body's lymphocytes, in facilitating immune cell trafficking, and direct immune modulatory functions, among others, the lymphatic system plays multifaceted roles in immune regulation. In this review, the potential for biomaterials to be applied to regulate the lymphatic system and its functions to achieve immunomodulation and the treatment of disease are described. Three related processes-lymphangiogenesis, lymphatic vessel contraction, and lymph node remodeling-are specifically explored. The molecular regulation of each process and their roles in pathologies are briefly outlined, with putative therapeutic targets and the lymphatic remodeling that can result from disease highlighted. Applications of biomaterials that harness these pathways for the treatment of disease via immunomodulation are discussed.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
