Cathepsin B Dependent Cleavage Product of Serum Amyloid A1 Identifies Patients with Chemotherapy-Related Cardiotoxicity
- PMID: 32259067
- PMCID: PMC7089022
- DOI: 10.1021/acsptsci.9b00035
Cathepsin B Dependent Cleavage Product of Serum Amyloid A1 Identifies Patients with Chemotherapy-Related Cardiotoxicity
Abstract
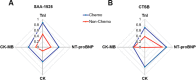
Improvements in long-term cancer survival rates have resulted in an increase in the prevalence of chemotherapy-linked cardiac failure, but treatment-induced cardiac injuries may not be detected until long after therapy. Monitoring cardiac function is recommended; however, cardiovascular injury in cancer patients differs from those with primary cardiac dysfunction, which limits the utility of traditional cardiac biomarkers. Here we examined plasma levels of peptides produced by cathepsin B, which is released during chemotherapy-induced cardiac injury. We applied nanotrap fractionation to enrich plasma peptides from cancer patients treated with or without chemotherapy. Peptides associated with chemotherapy-induced cardiotoxicity, but not other cardiac injury, were identified by mass spectrometry, and their dependence on cathepsin B activity was determined using enzyme inhibition experiments. We found that a peptide (SAA-1525) derived from serum amyloid A1 was significantly increased in cardiotoxicity patients, and its production was inhibited when plasma samples were pretreated with cathepsin B specific inhibitors. Plasma SAA-1525 also correlated with other markers of cardiac injury. Analysis of plasma SAA-1525 levels may hold potential as a rapid and minimally invasive method to monitor subclinical injury, thereby allowing timely intervention to mitigate further cardiac damage and avoid more severe clinical presentation.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Xu J.; Murphy S. L.; Kochanek K. D.; Bastian B. A. (2016) Deaths: Final Data for 2013. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System 64 (2), 1–119. - PubMed
-
- Minichillo S.; Gallelli I.; Barbieri E.; Cubelli M.; Rubino D.; Quercia S.; Dall’Olio M.; Rapezzi C.; Zamagni C. (2017) Trastuzumab resumption after extremely severe cardiotoxicity in metastatic breast cancer patient: a case report. BMC Cancer 17 (1), 722. 10.1186/s12885-017-3712-8. - DOI - PMC - PubMed
-
- Felker G. M.; Thompson R. E.; Hare J. M.; Hruban R. H.; Clemetson D. E.; Howard D. L.; Baughman K. L.; Kasper E. K. (2000) Underlying Causes and Long-Term Survival in Patients with Initially Unexplained Cardiomyopathy. N. Engl. J. Med. 342 (15), 1077–1084. 10.1056/NEJM200004133421502. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
