Antibody-Mediated Delivery of VEGFC Ameliorates Experimental Chronic Colitis
- PMID: 32259068
- PMCID: PMC7088901
- DOI: 10.1021/acsptsci.9b00037
Antibody-Mediated Delivery of VEGFC Ameliorates Experimental Chronic Colitis
Abstract
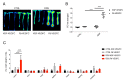
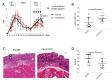
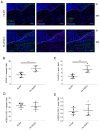
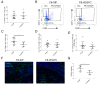
Crohn's disease (CD) and ulcerative colitis (UC) are two distinct forms of inflammatory bowel disease (IBD) characterized by an expanded lymphatic network with impaired functionality both in mouse models and in human patients. In this study, we investigated whether targeted delivery of the pro-lymphangiogenic vascular endothelial growth factor C (VEGFC) to the site of inflammation may represent a new, clinically feasible strategy for treating IBD. To achieve targeting of inflamed tissue, we developed a fusion protein consisting of human VEGFC fused to the F8 antibody (F8-VEGFC), which specifically binds to the extradomain A (EDA) of fibronectin, a spliced isoform almost exclusively expressed in inflamed tissues. The therapeutic activity of intravenously administered F8-VEGFC, compared to a targeted construct lacking VEGFC (F8-SIP), was investigated in a mouse model of dextran sodium sulfate (DSS)-induced colitis. The presence of EDA fibronectin was detected in both human and mouse inflamed colon tissue. Biodistribution studies of radiolabeled F8-VEGFC revealed a specific accumulation of the antibody in the colon of DSS-administered mice, as compared to an untargeted VEGFC fusion protein (KSF-VEGFC) (binding the irrelevant hen egg lysozyme antigen). Systemic treatment with F8-VEGFC significantly reduced the clinical and histological signs of inflammation, expanded the lymphatic vascular network, reduced the density of immune cells, and also decreased the expression of inflammatory cytokines in the inflamed colon. Overall, these results reveal that administration of F8-VEGFC represents a novel and promising approach for the treatment of IBD.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): D.N. is a shareholder and board member of Philogen, a biotech company which shares with ETH Zurich commercialization rights for F8-VEGFC. M.D., D.N., and S.S. are inventors on a pending patent for F8-VEGF-C. The other authors declare no competing financial interest.
Figures


References
-
- Geleff S.; Schoppmann S. F.; Oberhuber G. (2003) Increase in podoplanin-expressing intestinal lymphatic vessels in inflammatory bowel disease. Virchows Arch 442, 231–237. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
