Emerging Cystic Fibrosis Transmembrane Conductance Regulator Modulators as New Drugs for Cystic Fibrosis: A Portrait of in Vitro Pharmacology and Clinical Translation
- PMID: 32259083
- PMCID: PMC7088950
- DOI: 10.1021/acsptsci.9b00060
Emerging Cystic Fibrosis Transmembrane Conductance Regulator Modulators as New Drugs for Cystic Fibrosis: A Portrait of in Vitro Pharmacology and Clinical Translation
Abstract
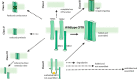
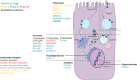
Pharmacological correction of the defective ion channel with cystic fibrosis transmembrane conductance regulator (CFTR) has become an attractive approach to therapy directed at the root cause of the life-limiting disease cystic fibrosis (CF). CFTR defects range from absence, misfolding, and resulting degradation to functional defects of the CFTR protein. The discovery and development of the CFTR potentiator ivacaftor was a major break-through in CF therapy and has triggered an enormous incentive for seeking effective modulators such as lumacaftor, tezacaftor or elexacaftor for all patients with CF. A number of emerging CFTR modulators are currently in the development pipeline, and rescue levels of CFTR protein approach a cure for cystic fibrosis. In this review, we identify and characterize all preclinical and clinical emerging CFTR modulators and discuss the in vitro pharmacology, looking at CFTR protein expression and chloride transport and the translation to the clinic. The new emerging CFTR modulators could offer new therapeutic solutions for CF patients.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
