Effect of Flumazenil on Hypoactive Delirium in the ICU: A Double-Blind, Placebo-Controlled Pilot Study
- PMID: 32259108
- PMCID: PMC7098541
- DOI: 10.1097/CCE.0000000000000085
Effect of Flumazenil on Hypoactive Delirium in the ICU: A Double-Blind, Placebo-Controlled Pilot Study
Abstract
To determine whether the use of flumazenil reverses hypoactive delirium and increases delirium-free days in critically ill patients who were exposed to benzodiazepine therapy during the ICU admission.
Design: This was a single-center, double-blinded, randomized placebo-controlled pilot study.
Setting: Adult ICUs at a large academic medical center in the United States.
Patients: Adult, critically ill patients with benzodiazepine exposure and hypoactive delirium based on the Confusion Assessment Method-ICU and Richmond Agitation Sedation Scale assessments were considered for enrollment.
Interventions: Patients received a test dose of flumazenil starting at 0.1 mg intravenously and titrated up every 5 minutes by 0.1 mg increments up to a maximum total dose of 2 mg. Patients who demonstrated a Richmond Agitation Sedation Scale score increase of greater than 1 point were considered responders and randomized to flumazenil (0.05-0.3 mg/hr) or placebo infusion for up to 72 hours. Confusion Assessment Method-ICU scores were assessed twice daily for resolution of delirium.
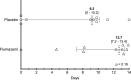
Measurements and main results: The trial was stopped early based on the observed size effect and power analysis. Twenty-two of the 25 patients responded to the flumazenil test dose (88%). The median number of delirium-free days alive without coma within 14 days of enrollment was similar between the two infusion groups (12.7 vs 9.2; p = 0.19). There was no difference in the probability of delirium resolution within the first 14 days with 90% versus 70% in the flumazenil and placebo groups, respectively (p = 0.2). There was no statistical difference (odds ratio, 0.17; 95% CI, 0.022-1.23; p = 0.079) in delirium- and coma-free days at the end of the study drug infusion. There was no difference between groups in ICU length of stay (7.8 ± 4.8 vs 7 ± 8; p = 0.74). No serious adverse events occurred.
Conclusions: This study found that flumazenil test dose and infusion present a potential option for hypoactive delirium associated with benzodiazepine exposure; however, the possible benefit is unknown. Larger studies are warranted to further evaluate these findings.
Keywords: benzodiazepine; benzodiazepine antagonist; critical care; delirium; flumazenil; hypoactive delirium.
Copyright © 2020 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Figures


References
-
- Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004; 291:1753–1762 - PubMed
-
- Patel SB, Poston JT, Pohlman A, et al. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014; 189:658–665 - PubMed
-
- Shehabi Y, Riker RR, Bokesch PM, et al. ; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010; 38:2311–2318 - PubMed

