Simple electrocardiographic measures improve sudden arrhythmic death prediction in coronary disease
- PMID: 32259257
- PMCID: PMC7263700
- DOI: 10.1093/eurheartj/ehaa177
Simple electrocardiographic measures improve sudden arrhythmic death prediction in coronary disease
Abstract
Aims: To determine whether the combination of standard electrocardiographic (ECG) markers reflecting domains of arrhythmic risk improves sudden and/or arrhythmic death (SAD) risk stratification in patients with coronary heart disease (CHD).
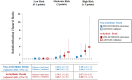
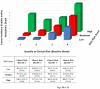
Methods and results: The association between ECG markers and SAD was examined in a derivation cohort (PREDETERMINE; N = 5462) with adjustment for clinical risk factors, left ventricular ejection fraction (LVEF), and competing risk. Competing outcome models assessed the differential association of ECG markers with SAD and competing mortality. The predictive value of a derived ECG score was then validated (ARTEMIS; N = 1900). In the derivation cohort, the 5-year cumulative incidence of SAD was 1.5% [95% confidence interval (CI) 1.1-1.9] and 6.2% (95% CI 4.5-8.3) in those with a low- and high-risk ECG score, respectively (P for Δ < 0.001). A high-risk ECG score was more strongly associated with SAD than non-SAD mortality (adjusted hazard ratios = 2.87 vs. 1.38 respectively; P for Δ = 0.003) and the proportion of deaths due to SAD was greater in the high vs. low risk groups (24.9% vs. 16.5%, P for Δ = 0.03). Similar findings were observed in the validation cohort. The addition of ECG markers to a clinical risk factor model inclusive of LVEF improved indices of discrimination and reclassification in both derivation and validation cohorts, including correct reclassification of 28% of patients in the validation cohort [net reclassification improvement 28 (7-49%), P = 0.009].
Conclusion: For patients with CHD, an externally validated ECG score enriched for both absolute and proportional SAD risk and significantly improved risk stratification compared to standard clinical risk factors including LVEF.
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT01114269. ClinicalTrials.gov ID NCT01114269.
Keywords: Coronary heart disease; Electrocardiogram; Sudden death.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2020. For permissions, please email: journals.permissions@oup.com.
Figures





Comment in
-
The electrocardiogram: are we at the dawn of a new era?Eur Heart J. 2020 Jun 1;41(21):2000-2002. doi: 10.1093/eurheartj/ehaa294. Eur Heart J. 2020. PMID: 32378699 No abstract available.
-
Identifying coronary artery disease patients at risk for sudden and/or arrhythmic death: remaining limitations of the electrocardiogram.Eur Heart J. 2020 Aug 7;41(30):2911-2912. doi: 10.1093/eurheartj/ehaa470. Eur Heart J. 2020. PMID: 32609337 No abstract available.
-
The electrocardiogram and sudden death: capturing electrical physiology and arrhythmic substrate.Eur Heart J. 2020 Aug 7;41(30):2911-2912. doi: 10.1093/eurheartj/ehaa472. Eur Heart J. 2020. PMID: 32609368 No abstract available.
-
Risk stratification refinement and implantable cardioverter defibrillator protection for the coronary artery disease patients with a preserved left ventricular systolic function with a two-step programmed ventricular stimulation-inclusive approach.Eur Heart J. 2021 Jan 21;42(4):355-356. doi: 10.1093/eurheartj/ehaa772. Eur Heart J. 2021. PMID: 33197239 No abstract available.
References
-
- Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 2006;47:1161–1166. - PubMed
-
- Aro AL, Reinier K, Rusinaru C, Uy-Evanado A, Darouian N, Phan D, Mack WJ, Jui J, Soliman EZ, Tereshchenko LG, Chugh SS. Electrical risk score beyond the left ventricular ejection fraction: prediction of sudden cardiac death in the Oregon Sudden Unexpected Death Study and the Atherosclerosis Risk in Communities Study. Eur Heart J 2017;38:3017–3025. - PMC - PubMed
-
- Waks JW, Sitlani CM, Soliman EZ, Kabir M, Ghafoori E, Biggs ML, Henrikson CA, Sotoodehnia N, Biering-Sorensen T, Agarwal SK, Siscovick DS, Post WS, Solomon SD, Buxton AE, Josephson ME, Tereshchenko LG. Global electric heterogeneity risk score for prediction of sudden cardiac death in the general population: the Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Circulation 2016;133:2222–2234. - PMC - PubMed
-
- Elming MB, Nielsen JC, Haarbo J, Videbæk L, Korup E, Signorovitch J, Olesen LL, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbæk R, Hassager C, Svendsen JH, Høfsten DE, Torp-Pedersen C, Pehrson S, Køber L, Thune JJ. Age and outcomes of primary prevention implantable cardioverter-defibrillators in patients with nonischemic systolic heart failure. Circulation 2017;136:1772–1780. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

