Ngly1 -/- rats develop neurodegenerative phenotypes and pathological abnormalities in their peripheral and central nervous systems
- PMID: 32259258
- PMCID: PMC7322575
- DOI: 10.1093/hmg/ddaa059
Ngly1 -/- rats develop neurodegenerative phenotypes and pathological abnormalities in their peripheral and central nervous systems
Abstract
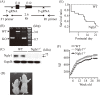
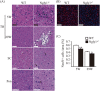
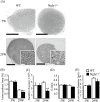
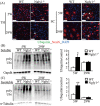
N-glycanase 1 (NGLY1) deficiency, an autosomal recessive disease caused by mutations in the NGLY1 gene, is characterized by developmental delay, hypolacrima or alacrima, seizure, intellectual disability, movement disorders and other neurological phenotypes. Because of few animal models that recapitulate these clinical signatures, the mechanisms of the onset of the disease and its progression are poorly understood, and the development of therapies is hindered. In this study, we generated the systemic Ngly1-deficient rodent model, Ngly1-/- rats, which showed developmental delay, movement disorder, somatosensory impairment and scoliosis. These phenotypes in Ngly1-/- rats are consistent with symptoms in human patients. In accordance with the pivotal role played by NGLY1 in endoplasmic reticulum-associated degradation processes, cleaving N-glycans from misfolded glycoproteins in the cytosol before they can be degraded by the proteasome, loss of Ngly1 led to accumulation of cytoplasmic ubiquitinated proteins, a marker of misfolded proteins in the neurons of the central nervous system of Ngly1-/- rats. Histological analysis identified prominent pathological abnormalities, including necrotic lesions, mineralization, intra- and extracellular eosinophilic bodies, astrogliosis, microgliosis and significant loss of mature neurons in the thalamic lateral and the medial parts of the ventral posterior nucleus and ventral lateral nucleus of Ngly1-/- rats. Axonal degradation in the sciatic nerves was also observed, as in human subjects. Ngly1-/- rats, which mimic the symptoms of human patients, will be a useful animal model for preclinical testing of therapeutic options and understanding the detailed mechanisms of NGLY1 deficiency.
© The Author(s) 2020. Published by Oxford University Press.
Figures



References
-
- Huang C., Harada Y., Hosomi A., Masahara-Negishi Y., Seino J., Fujihira H., Funakoshi Y., Suzuki T., Dohmae N. and Suzuki T. (2015) Endo-β-N-acetylglucosaminidase forms N-GlcNAc protein aggregates during ER-associated degradation in Ngly1-defective cells. Proc. Natl. Acad. Sci. U. S. A., 112, 1398–1403. - PMC - PubMed
-
- Suzuki T. (2015) The cytoplasmic peptide:N-glycanase (Ngly1)-basic science encounters a human genetic disorder. J. Biochem., 157, 23–24. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

