The Ah Receptor: Adaptive Metabolism, Ligand Diversity, and the Xenokine Model
- PMID: 32259433
- PMCID: PMC7175458
- DOI: 10.1021/acs.chemrestox.9b00476
The Ah Receptor: Adaptive Metabolism, Ligand Diversity, and the Xenokine Model
Abstract
The Ah receptor (AHR) has been studied for almost five decades. Yet, we still have many important questions about its role in normal physiology and development. Moreover, we still do not fully understand how this protein mediates the adverse effects of a variety of environmental pollutants, such as the polycyclic aromatic hydrocarbons (PAHs), the chlorinated dibenzo-p-dioxins ("dioxins"), and many polyhalogenated biphenyls. To provide a platform for future research, we provide the historical underpinnings of our current state of knowledge about AHR signal transduction, identify a few areas of needed research, and then develop concepts such as adaptive metabolism, ligand structural diversity, and the importance of proligands in receptor activation. We finish with a discussion of the cognate physiological role of the AHR, our perspective on why this receptor is so highly conserved, and how we might think about its cognate ligands in the future.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


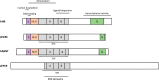

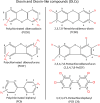

References
-
- Conney A. H.; et al. (1960) Adaptive increases in drug-metabolizing enzymes induced by phenobarbital and other drugs. Journal of Pharmacology and Experimental Therapeutics 130 (1), 1–8. - PubMed
-
- Hoffman D.; et al. (1972) Chemical studies on tobacco-smoke. 16. Fluoranthenes - quantitative determination in cigarette-smoke, formation by pyrolysis, and tumor-initiating activity. Journal of the National Cancer Institute 49 (4), 1165–1175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

