An Airway Protection Program Revealed by Sweeping Genetic Control of Vagal Afferents
- PMID: 32259485
- PMCID: PMC7197391
- DOI: 10.1016/j.cell.2020.03.004
An Airway Protection Program Revealed by Sweeping Genetic Control of Vagal Afferents
Abstract
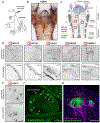
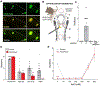
Sensory neurons initiate defensive reflexes that ensure airway integrity. Dysfunction of laryngeal neurons is life-threatening, causing pulmonary aspiration, dysphagia, and choking, yet relevant sensory pathways remain poorly understood. Here, we discover rare throat-innervating neurons (∼100 neurons/mouse) that guard the airways against assault. We used genetic tools that broadly cover a vagal/glossopharyngeal sensory neuron atlas to map, ablate, and control specific afferent populations. Optogenetic activation of vagal P2RY1 neurons evokes a coordinated airway defense program-apnea, vocal fold adduction, swallowing, and expiratory reflexes. Ablation of vagal P2RY1 neurons eliminates protective responses to laryngeal water and acid challenge. Anatomical mapping revealed numerous laryngeal terminal types, with P2RY1 neurons forming corpuscular endings that appose laryngeal taste buds. Epithelial cells are primary airway sentinels that communicate with second-order P2RY1 neurons through ATP. These findings provide mechanistic insights into airway defense and a general molecular/genetic roadmap for internal organ sensation by the vagus nerve.
Keywords: cough; interoception; larynx; nodose ganglion; optogenetics; peripheral nervous system; petrosal ganglion; purinergic receptors; single cell RNA sequencing; superior laryngeal nerve.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests S.D.L. is a consultant for Kallyope.
Figures




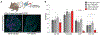
Comment in
-
Airway Protection-A Role for Vagal P2RY1 Receptors.Cell. 2020 Apr 30;181(3):509-511. doi: 10.1016/j.cell.2020.04.006. Cell. 2020. PMID: 32359432
-
Commentary: Vagal P2RY1 Receptors: A Novel Target for Airway Disease.Front Pharmacol. 2020 Nov 30;11:596003. doi: 10.3389/fphar.2020.596003. eCollection 2020. Front Pharmacol. 2020. PMID: 33390983 Free PMC article. No abstract available.
References
-
- Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, and Smith JA (2015). P2×3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 385, 1198–1205. - PubMed
-
- Anderson JW, Sant’Ambrogio FB, Mathew OP, and Sant’Ambrogio G (1990). Water-responsive laryngeal receptors in the dog are not specialized endings. Respir Physiol 79, 33–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

