Identification, clinical manifestation and structural mechanisms of mutations in AMPK associated cardiac glycogen storage disease
- PMID: 32259713
- PMCID: PMC7132172
- DOI: 10.1016/j.ebiom.2020.102723
Identification, clinical manifestation and structural mechanisms of mutations in AMPK associated cardiac glycogen storage disease
Abstract
Background: Although 21 causative mutations have been associated with PRKAG2 syndrome, our understanding of the syndrome remains incomplete. The aim of this project is to further investigate its unique genetic background, clinical manifestations, and underlying structural changes.
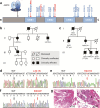
Methods: We recruited 885 hypertrophic cardiomyopathy (HCM) probands and their families internationally. Targeted next-generation sequencing of sudden cardiac death (SCD) genes was performed. The role of the identified variants was assessed using histological techniques and computational modeling.
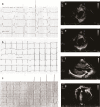
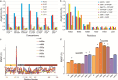
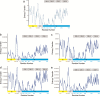
Findings: Twelve PRKAG2 syndrome kindreds harboring 5 distinct variants were identified. The clinical penetrance of 25 carriers was 100.0%. Twenty-two family members died of SCD or heart failure (HF). All probands developed bradycardia (HRmin, 36.3 ± 9.8 bpm) and cardiac conduction defects, and 33% had evidence of atrial fibrillation/paroxysmal supraventricular tachycardia (PSVT) and 67% had ventricular preexcitation, respectively. Some carriers presented with apical hypertrophy, hypertension, hyperlipidemia, and renal insufficiency. Histological study revealed reduced AMPK activity and major cardiac channels in the heart tissue with K485E mutation. Computational modelling suggests that K485E disrupts the salt bridge connecting the β and γ subunits of AMPK, R302Q/P decreases the binding affinity for ATP, T400N and H401D alter the orientation of H383 and R531 residues, thus altering nucleotide binding, and N488I and L341S lead to structural instability in the Bateman domain, which disrupts the intramolecular regulation.
Interpretation: Including 4 families with 3 new mutations, we describe a cohort of 12 kindreds with PRKAG2 syndrome with novel pathogenic mechanisms by computational modelling. Severe clinical cardiac phenotypes may be developed, including HF, requiring close follow-up.
Keywords: Arrhythmia; Cardiomyopathy; Genetics; Heart failure; PRKAG2 syndrome; Sudden cardiac death.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors report no relationships that could be construed as a conflict of interest.
Figures



Comment in
-
Too much sugar leaves a sour taste: A cardiac disease caused by excess glycogen deposit.EBioMedicine. 2020 May;55:102764. doi: 10.1016/j.ebiom.2020.102764. Epub 2020 Apr 24. EBioMedicine. 2020. PMID: 32339940 Free PMC article. No abstract available.
References
-
- Gersh B.J., Maron B.J., Bonow R.O., Dearani J.A., Fifer M.A., Link M.S. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. 2011;124(24):e783–e831. - PubMed
-
- Authors/Task Force m, Elliott P.M., Anastasakis A., Borger M.A., Borggrefe M., Cecchi F. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the european society of cardiology (ESC) Eur Heart J. 2014;35(39):2733–2779. - PubMed
-
- Gollob M.H., Seger J.J., Gollob T.N., Tapscott T., Gonzales O., Bachinski L. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation. 2001;104(25):3030–3033. - PubMed
-
- Gollob M.H., Roberts R. AMP-activated protein kinase and familial Wolff-Parkinson-White syndrome: new perspectives on heart development and arrhythmogenesis. Eur Heart J. 2002;23(9):679–681. - PubMed
-
- Sidhu J.S., Rajawat Y.S., Rami T.G., Gollob M.H., Wang Z., Yuan R. Transgenic mouse model of ventricular preexcitation and atrioventricular reentrant tachycardia induced by an AMP-activated protein kinase loss-of-function mutation responsible for wolff-parkinson-white syndrome. Circulation. 2005;111(1):21–29. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

