Dual luminescent charge transfer probe for quantitative detection of serum albumin in aqueous samples
- PMID: 32259717
- PMCID: PMC7196023
- DOI: 10.1016/j.saa.2020.118305
Dual luminescent charge transfer probe for quantitative detection of serum albumin in aqueous samples
Abstract
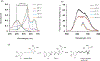
In diagnostic medicine serum albumin is considered as an important biomarker for assessment of cardiovascular functions and diagnosis of renal diseases. Herein, we report a novel donor-π-π-acceptor fluorophore for selective detection of serum albumin in urine samples. In our design, a phenolic donor was conjugated with a tricyanofuran (TCF) acceptor through a dimethine bridge via a simple condensation reaction. The stereoelectronic effects of the incorporated methoxy (-OCH3) groups and the TCF moiety-in conjunction with the extended π-electron conjugation-led to dual red and NIR-I absorption/emission in water. Moreover, due to superior electron transfer between a phenolate donor and the TCF acceptor and the subsequent energy decay from the charge transfer states, the fluorophore displayed negligible fluorescence emission in water and other polar solvents. Consequently, we have been able to utilize the fluorophore for quantitative estimation of serum albumin both in the red (<700 nm) and NIR-I (700-900 nm) regions of the electromagnetic spectrum with excellent reproducibility. The fluorophore selectively recognized human serum albumin over other proteins and enzymes with a limit of detection of 10 mg/L and 20 mg/L in simulated urine samples at red and NIR-I emission window of the spectrum, respectively. By molecular docking analysis and experimental displacement assays, we have shown that the selective response of the fluorophore toward human serum albumin is due to tighter supramolecular complexation between the fluorophore and the protein at subdomain IB, and the origin of the NIR-I (780 nm) emission was attributed to a twisted conformer of phenolate-π-π-TCF system in aqueous solution. These findings indicate that the fluorophore could be utilized for quantitative detection of human serum albumin in urine samples for clinical diagnosis of albuminuria.
Keywords: Dual emission probe; Human serum albumin; Microalbuminuria; Near infrared emission; Selective protein recognition.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

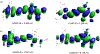



References
-
- Quinlan GJ, Martin GS, Evans TW, Hepatology, 2005, 41, 1211–1219. - PubMed
-
- Peters T, All about albumin: biochemistry, genetics, and medical applications. Academic Press Inc., San Diego, USA, 1996.
-
- Doumas BT, Peters T, Clin. Chim. Acta, 1997, 258, 3–20. - PubMed
-
- Arques S, Ambrosi P, Card J. Failure, 2011, 17, 451–458. - PubMed
-
- Hoogenberg K, Sluiter WJ, Dullaart RP, Acta Endocrinol, 1993, 129, 151–157. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

