Female AhR Knockout Mice Develop a Minor Renal Insufficiency in an Adenine-Diet Model of Chronic Kidney Disease
- PMID: 32260098
- PMCID: PMC7177716
- DOI: 10.3390/ijms21072483
Female AhR Knockout Mice Develop a Minor Renal Insufficiency in an Adenine-Diet Model of Chronic Kidney Disease
Abstract
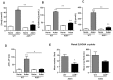
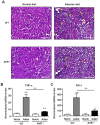
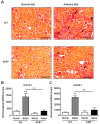
Cardiovascular complications observed in chronic kidney disease (CKD) are associated with aryl hydrocarbon receptor (AhR) activation by tryptophan-derived uremic toxins-mainly indoxyl sulfate (IS). AhR is a ligand-activated transcription factor originally characterized as a receptor of xenobiotics involved in detoxification. The aim of this study was to determine the role of AhR in a CKD mouse model based on an adenine diet. Wild-type (WT) and AhR-/- mice were fed by alternating an adenine-enriched diet and a regular diet for 6 weeks. Our results showed an increased mortality rate of AhR-/- males. AhR-/- females survived and developed a less severe renal insufficiency that WT mice, reflected by urea, creatinine, and IS measurement in serum. The protective effect was related to a decrease of pro-inflammatory and pro-fibrotic gene expression, an attenuation of tubular injury, and a decrease of 2,8-dihydroxyadenine crystal deposition in the kidneys of AhR-/- mice. These mice expressed low levels of xanthine dehydrogenase, which oxidizes adenine into 2,8-dihydroxyadenine, and low levels of the IS metabolism enzymes. In conclusion, the CKD model of adenine diet is not suitable for AhR knockout mice when studying the role of this transcription factor in cardiovascular complications, as observed in human CKD.
Keywords: adenine; aryl hydrocarbon receptor, indoxyl sulfate; chronic kidney disease; mouse model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Villain C., Metzger M., Combe C., Fouque D., Frimat L., Jacquelinet C., Laville M., Briancon S., Klein J., Schanstra J.P., et al. Prevalence of atheromatous and non-atheromatous cardiovascular disease by age in chronic kidney disease. Nephrol. Dial. Transplant. 2018 doi: 10.1093/ndt/gfy277. - DOI - PubMed
-
- Barreto F.C., Barreto D.V., Liabeuf S., Meert N., Glorieux G., Temmar M., Choukroun G., Vanholder R., Massy Z.A. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. - DOI - PMC - PubMed
-
- Sallee M., Dou L., Cerini C., Poitevin S., Brunet P., Burtey S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: A new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel) 2014;6:934–949. doi: 10.3390/toxins6030934. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

