Sodium Imbalance in Mice Results Primarily in Compensatory Gene Regulatory Responses in Kidney and Colon, but Not in Taste Tissue
- PMID: 32260115
- PMCID: PMC7230584
- DOI: 10.3390/nu12040995
Sodium Imbalance in Mice Results Primarily in Compensatory Gene Regulatory Responses in Kidney and Colon, but Not in Taste Tissue
Abstract
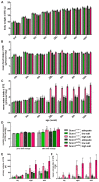
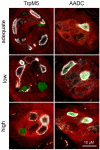
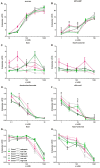
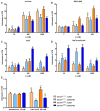
Renal excretion and sodium appetite provide the basis for sodium homeostasis. In both the kidney and tongue, the epithelial sodium channel (ENaC) is involved in sodium uptake and sensing. The diuretic drug amiloride is known to block ENaC, producing a mild natriuresis. However, amiloride is further reported to induce salt appetite in rodents after prolonged exposure as well as bitter taste impressions in humans. To examine how dietary sodium content and amiloride impact on sodium appetite, mice were subjected to dietary salt and amiloride intervention and subsequently analyzed for ENaC expression and taste reactivity. We observed substantial changes of ENaC expression in the colon and kidney confirming the role of these tissues for sodium homeostasis, whereas effects on lingual ENaC expression and taste preferences were negligible. In comparison, prolonged exposure to amiloride-containing drinking water affected β- and αENaC expression in fungiform and posterior taste papillae, respectively, next to changes in salt taste. However, amiloride did not only change salt taste sensation but also perception of sucrose, glutamate, and citric acid, which might be explained by the fact that amiloride itself activates bitter taste receptors in mice. Accordingly, exposure to amiloride generally affects taste impression and should be evaluated with care.
Keywords: amiloride; epithelial sodium channel; salt deprivation; short-term preference test; sodium homeostasis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Loffing J., Pietri L., Aregger F., Bloch-Faure M., Ziegler U., Meneton P., Rossier B.C., Kaissling B. Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am. J. Physiol. Ren. Physiol. 2000;279:F252–258. doi: 10.1152/ajprenal.2000.279.2.F252. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

