Small Non-Coding RNA Profiling Identifies miR-181a-5p as a Mediator of Estrogen Receptor Beta-Induced Inhibition of Cholesterol Biosynthesis in Triple-Negative Breast Cancer
- PMID: 32260128
- PMCID: PMC7226848
- DOI: 10.3390/cells9040874
Small Non-Coding RNA Profiling Identifies miR-181a-5p as a Mediator of Estrogen Receptor Beta-Induced Inhibition of Cholesterol Biosynthesis in Triple-Negative Breast Cancer
Abstract
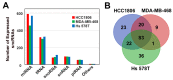
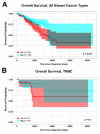
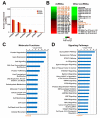
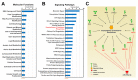
Triple-negative breast cancer (TNBC) is a highly heterogeneous disease, representing the most aggressive breast cancer (BC) subtype with limited treatment options due to a lack of estrogen receptor alpha (ERα), progesterone receptor (PR), and Erb-B2 receptor tyrosine kinase 2 (HER2/neu) expression. Estrogen receptor beta (ERβ) is present in a fraction of TNBC patients, where its expression correlates with improved patient outcomes, supported by the fact that it exerts oncosuppressive effects in TNBC cell models in vitro. ERβ is involved in microRNA-mediated regulation of gene expression in hormone-responsive BC cells and could mediate its actions through small noncoding RNAs (sncRNAs) in TNBCs also. To verify this possibility, smallRNA sequencing was performed on three ERβ-expressing cell lines from different TNBC molecular subtypes. Several sncRNAs resulted modulated by ERβ, with a subset being regulated in a tumor subtype-independent manner. Interestingly, sncRNA profiling of 12 ERβ+and 32 ERβ- primary TNBC biopsies identified 7 microRNAs, 1 PIWI-interacting RNA (piRNA), and 1 transfer RNA (tRNA) differentially expressed in ERβ+ compared to ERβ- tumors and cell lines. Among them, miR-181a-5p was found to be overexpressed in ERβ+ tumors and predicted target key components of the cholesterol biosynthesis pathway previously found to be inhibited by ERβ in TNBC cells.
Keywords: cholesterol biosynthesis; estrogen receptor beta; microRNA; small non-coding RNAs; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

