Ranunculus bulumei Methanol Extract Exerts Anti-Inflammatory Activity by Targeting Src/Syk in NF-κB Signaling
- PMID: 32260181
- PMCID: PMC7226355
- DOI: 10.3390/biom10040546
Ranunculus bulumei Methanol Extract Exerts Anti-Inflammatory Activity by Targeting Src/Syk in NF-κB Signaling
Abstract
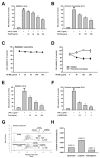
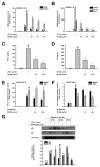
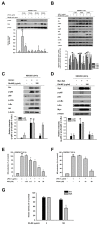
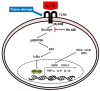
(1) Background: Ranunculus bulumei is a flowering plant that belongs to the Ranunculus species. Several Ranunculus species, such as R. aquatilis and R. muricatus, have traditionally been used to treat fever and rheumatism throughout Asia, suggesting that plants belonging to the Ranunculus species may have anti-inflammatory effects. To our knowledge, the pharmacological activity of R. bulumei has not been reported. Therefore, in this study, we aim to assess the anti-inflammatory activity of a methanol extract that was derived from R. bulumei (Rb-ME) in macrophage-mediated inflammatory responses and to identify the molecular mechanism that underlies any anti-inflammatory action. (2) Methods: The anti-inflammatory efficacy of Rb-ME was evaluated while using in vitro and in vivo experiments. The RAW264.7 cells and peritoneal macrophages were stimulated by lipopolysaccharide (LPS). In addition, LPS-induced peritonitis and HCl/EtOH-triggered gastritis models were produced. A nitric oxide (NO) assay, real-time PCR, luciferase reporter gene assay, western blot analysis, plasmid overexpression strategy, and in vitro kinase assay were used to determine the molecular mechanisms and target molecules of Rb-ME. The phytochemical active ingredients of Rb-ME were also identified by high performance liquid chromatograph (HPLC). (3) Results: Rb-ME reduced the production of NO and mRNA expression of iNOS, COX-2, IL-1β, and IL-6 without cytotoxicity. The protein secretion of TNF-α and IL-6 was also decreased by Rb-ME. HPLC analysis indicates that quercetin, luteolin, and kaempferol are the main active ingredients in the anti-inflammatory efficacy of Rb-ME. Rb-ME also blocked MyD88-induced NF-κB promoter activity and nuclear translocation of NF-κB subunits (p65 and p50). Moreover, Rb-ME reduced the phosphorylation of IκBα, Akt, p85, Src, and Syk, which are NF-κB upstream signaling molecules in LPS-activated RAW264.7 cells. According to the in vitro kinase assay, Rb-ME directly inhibits Syk kinase activity. The oral administration of Rb-ME alleviated inflammatory responses and the levels of p-IκBα in mice with LPS-induced peritonitis and HCl/EtOH-induced gastritis. (4) Conclusions Rb-ME has anti-inflammatory capacity by suppressing NF-κB signaling and it has been found to target Src and Syk in the NF-κB pathway. Based on this efficacy, Rb-ME could be developed as an anti-inflammatory herbal medicine.
Keywords: NF-κB signal pathway; Ranunculus bulumei; Src; Syk; anti-inflammatory activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

