Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis
- PMID: 32260219
- PMCID: PMC7226834
- DOI: 10.3390/cells9040880
Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis
Abstract
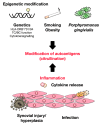
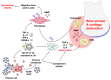
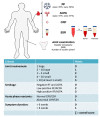
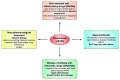
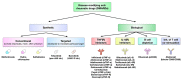
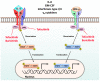
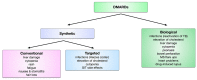
Rheumatoid arthritis (RA) is an autoimmune disease that involves multiple joints bilaterally. It is characterized by an inflammation of the tendon (tenosynovitis) resulting in both cartilage destruction and bone erosion. While until the 1990s RA frequently resulted in disability, inability to work, and increased mortality, newer treatment options have made RA a manageable disease. Here, great progress has been made in the development of disease-modifying anti-rheumatic drugs (DMARDs) which target inflammation and thereby prevent further joint damage. The available DMARDs are subdivided into (1) conventional synthetic DMARDs (methotrexate, hydrochloroquine, and sulfadiazine), (2) targeted synthetic DMARDs (pan-JAK- and JAK1/2-inhibitors), and (3) biologic DMARDs (tumor necrosis factor (TNF)-α inhibitors, TNF-receptor (R) inhibitors, IL-6 inhibitors, IL-6R inhibitors, B cell depleting antibodies, and inhibitors of co-stimulatory molecules). While DMARDs have repeatedly demonstrated the potential to greatly improve disease symptoms and prevent disease progression in RA patients, they are associated with considerable side-effects and high financial costs. This review summarizes our current understanding of the underlying pathomechanism, diagnosis of RA, as well as the mode of action, clinical benefits, and side-effects of the currently available DMARDs.
Keywords: IL-6; TNF; autoimmunity; rheumatoid arthritis.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

