Azides and Porphyrinoids: Synthetic Approaches and Applications. Part 1-Azides, Porphyrins and Corroles
- PMID: 32260294
- PMCID: PMC7181322
- DOI: 10.3390/molecules25071662
Azides and Porphyrinoids: Synthetic Approaches and Applications. Part 1-Azides, Porphyrins and Corroles
Abstract
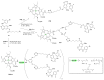
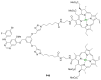
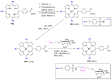
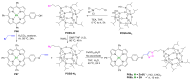
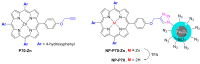
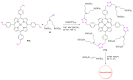
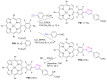
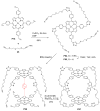
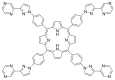
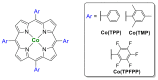
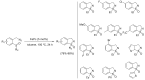
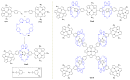
Azides and porphyrinoids (such as porphyrin and corrole macrocycles) can give rise to new derivatives with significant biological properties and as new materials' components. Significant synthetic approaches have been studied. A wide range of products (e.g., microporous organic networks, rotaxane and dendritic motifs, dendrimers as liquid crystals, as blood substitutes for transfusions and many others) can now be available and used for several medicinal and industrial purposes.
Keywords: Azides; Catalysis; Click Chemistry; Corroles; Cycloadditions; Microorganisms Photoinactivation; Photodynamic Therapy; Porphyrinoids; Porphyrins; Supramolecular assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


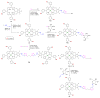


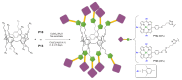

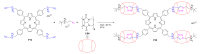


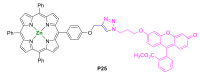




































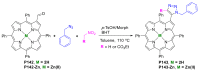

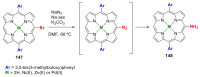
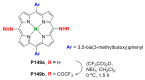

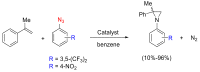
















References
-
- Griess J.P.X.X. On a new class of compounds in which nitrogen is substituted for hydrogen. Proc. R. Soc. Lond. 1864;13:375–384.
-
- Curtius T. Ueber Stickstoffwasserstoffsäure (Azoimid) N3H. Ber. Dtsch. Chem. Ges. 1890;23:3023–3033. doi: 10.1002/cber.189002302232. - DOI
-
- Lerner L. Small-Scale Synthesis of Laboratory Reagents with Reaction Modeling. 1st ed. CRC Press; Boca Raton, FL, USA: 2011.
-
- Pinho e Melo T.M.V.D. Synthesis of Azides. In: Bräse S., Banert K., editors. Organic Azides: Syntheses and Applications. John Wiley & Sons, Ltd; Chichester, UK: 2009. pp. 53–94.
-
- Schilling C., Jung N., Bräse S. Cycloaddition Reactions with Azides: An Overview. In: Bräse S., Banert K., editors. Organic Azides: Syntheses and Applications. John Wiley & Sons, Ltd; Chichester, UK: 2009. pp. 269–284.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

