Major Depressive Disorder is Associated with Impaired Mitochondrial Function in Skin Fibroblasts
- PMID: 32260327
- PMCID: PMC7226727
- DOI: 10.3390/cells9040884
Major Depressive Disorder is Associated with Impaired Mitochondrial Function in Skin Fibroblasts
Abstract
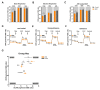
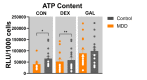
Mitochondrial malfunction is supposed to be involved in the etiology and pathology of major depressive disorder (MDD). Here, we aimed to identify and characterize the molecular pathomechanisms related to mitochondrial dysfunction in adult human skin fibroblasts, which were derived from MDD patients or non-depressive control subjects. We found that MDD fibroblasts showed significantly impaired mitochondrial functioning: basal and maximal respiration, spare respiratory capacity, non-mitochondrial respiration and adenosine triphosphate (ATP)-related oxygen consumption was lower. Moreover, MDD fibroblasts harbor lower ATP levels and showed hyperpolarized mitochondrial membrane potential. To investigate cellular resilience, we challenged both groups of fibroblasts with hormonal (dexamethasone) or metabolic (galactose) stress for one week, and found that both stressors increased oxygen consumption but lowered ATP content in MDD as well as in non-depressive control fibroblasts. Interestingly, the bioenergetic differences between fibroblasts from MDD or non-depressed subjects, which were observed under non-treated conditions, could not be detected after stress. Our findings support the hypothesis that altered mitochondrial function causes a bioenergetic imbalance, which is associated with the molecular pathophysiology of MDD. The observed alterations in the oxidative phosphorylation system (OXPHOS) and other mitochondria-related properties represent a basis for further investigations of pathophysiological mechanisms and might open new ways to gain insight into antidepressant signaling pathways.
Keywords: adenosine triphosphate; bioenergetics; calcium imaging; major depression; mitochondria; mitochondrial DNA copy number; mitochondrial membrane potential; oxidative phosphorylation; skin fibroblasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

