Biofilm Production Ability, Virulence and Antimicrobial Resistance Genes in Staphylococcus aureus from Various Veterinary Hospitals
- PMID: 32260416
- PMCID: PMC7238219
- DOI: 10.3390/pathogens9040264
Biofilm Production Ability, Virulence and Antimicrobial Resistance Genes in Staphylococcus aureus from Various Veterinary Hospitals
Abstract
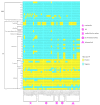
: Staphylococcus aureus (S. aureus) is one of the most clinically important zoonotic pathogens, but an understanding of the prevalence, biofilm formulation ability, virulence, and antimicrobial resistance genes of S. aureus from veterinary hospitals is lacking. By characterizing S. aureus in different origins of veterinary hospitals in Guangzhou, China, in 2019, we identified with the presence of S. aureus in pets (17.1%), veterinarians (31.7%), airborne dust (19.1%), environmental surfaces (4.3%), and medical device surfaces (10.8%). Multilocus sequence typing (MLST) and Staphylococcus protein A (spa) typing analyses demonstrated methicillin-sensitive S. aureus (MSSA) ST398-t571, MSSA ST188-t189, and methicillin-resistant S. aureus (MRSA) ST59-t437 were the most prevalent lineage. S. aureus with similar pulsed-field gel electrophoresis (PFGE) types distributed widely in different kinds of samples. The crystal violet straining assays revealed 100% (3/3) of MRSA ST59 and 81.8% (9/11) of MSSA ST188 showed strong biofilm formulation ability, whereas other STs (ST1, ST5, ST7, ST15, ST88, ST398, ST3154 and ST5353) showed weak biofilm production ability. Polymerase chain reaction (PCR) confirmed the most prevalent leucocidin, staphylococcal enterotoxins, ica operon, and adhesion genes were lukD-lukE (49.0%), sec-sel (15.7%), icaA-icaB-icaC-icaR (100.0%), and fnbB-cidA-fib-ebps-eno (100.0%), respectively. Our study showed that the isolates with strong biofilm production ability had a higher prevalence in clfA, clfB, fnbA and sdrC genes compared to the isolates with weak biofilm production ability. Furthermore, 2 ST1-MRSA isolates with tst gene and 1 ST88-MSSA isolate with lukS/F-PV gene were detected. In conclusion, the clonal dissemination of S. aureus of different origins in veterinary hospitals may have occurred; the biofilm production capacity of S. aureus is strongly correlated with ST types; some adhesion genes such as clfA, clfB, fnbA, and sdrC may pose an influence on biofilm production ability and the emergence of lukS/F-PV and tst genes in S. aureus from veterinary hospitals should raise our vigilance.
Keywords: Staphylococcus aureus; antimicrobial resistance; biofilm; prevalence; veterinary hospital; virulence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

