Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy
- PMID: 32264820
- PMCID: PMC7137484
- DOI: 10.1186/s10194-020-01102-9
Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy
Abstract
Background: We aimed to assess the efficacy and safety of erenumab, a fully human monoclonal antibody inhibiting the calcitonin gene-related peptide receptor (CGRPr), for the prevention of migraine in a real-life setting.
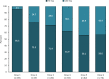
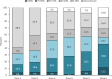
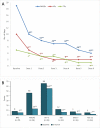
Main body: We included in our observational study all patients with episodic or chronic migraine treated with erenumab during the year 2019 in the Abruzzo region, central Italy, and with a 6-month follow-up. We included 89 patients; 76 (85.4%) received 6 doses of erenumab, 11 (12.4%) autonomously withdrew the drug due to perceived inefficacy, and 2 (2.2%) due to adverse events. Seventy-eight patients (87.6%) were female, with a mean age of 46.8 ± 11.2 years; 84 (94.4%) had chronic migraine, and 64 (71.9%) medication overuse. All patients had ≥2 prior preventive treatment failures. Fifty-three patients (69.7%) had a 50% decrease in monthly migraine days (MMDs) within the first three doses; 46 (71.9%) of 64 patients withdrew medication overuse. In the 76 patients who completed a 6-dose treatment, erenumab decreased median MMDs from 19 (interquartile range [IQR] 12-27.5) to 4 (IQR 2-9.5; P < 0.001), median monthly days of analgesic use from 10 (IQR 4.5-20) to 2 IQR 0-5; P < 0.001), and median monthly days of triptan use from 5 (IQR 0-15.5) to 1 (IQR 0-4; P < 0.001). We recorded 27 adverse events in 20 (22.5%) patients, the most common being constipation (13.5%). One adverse event, i.e. allergic reaction, led to treatment discontinuation in one patient.
Conclusions: Our real-life data confirm the efficacy and tolerability of erenumab for the prevention of migraine in a difficult-to-treat population of patients with a high prevalence of chronic migraine and medication overuse.
Keywords: Calcitonin gene-related peptide; Erenumab; Migraine; Migraine prevention; Monoclonal antibodies; Real-life study.
Conflict of interest statement
RO has received sponsorship to attend meetings from Novartis and Teva; SS had a financial relationship (lecturer or member of advisory board) with Abbott, Allergan, Novartis, Teva, and Eli Lilly; GA has received funds for congress participation from Innovet Italia Srl, Epitech Group and Lusofarmaco; MAG received funds for congress participation from IBSA; AC, IF, AG, MA, MM, FM, SV, DC, CM, and FP declare no competing interests.
Figures


References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
-
- Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the quality standards Subcommittee of the American Academy of neurology and the American headache society. Neurology. 2012;78(17):1337–1345. doi: 10.1212/WNL.0b013e3182535d20. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

