Strategy using a new antigenic test for rapid diagnosis of Streptococcus pneumoniae infection in respiratory samples from children consulting at hospital
- PMID: 32264834
- PMCID: PMC7137283
- DOI: 10.1186/s12866-020-01764-0
Strategy using a new antigenic test for rapid diagnosis of Streptococcus pneumoniae infection in respiratory samples from children consulting at hospital
Abstract
Background: Despite vaccination programs, Streptococcus pneumoniae remains among the main microorganisms involved in bacterial pneumonia, notably in terms of severity. The prognosis of pneumococcal infections is conditioned in part by the precocity of the diagnosis. The aim of this study was to evaluate the impact of a Rapid Diagnostic Test (RDT) targeting cell wall polysaccharide of Streptococcus pneumoniae and performed directly in respiratory samples, on the strategy of diagnosis of respiratory pneumococcal infections in children.
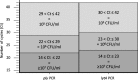
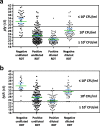
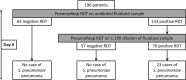
Results: Upper-respiratory tract samples from 196 children consulting at hospital for respiratory infection were tested for detecting S. pneumoniae using a newly-designed RDT (PneumoResp, Biospeedia), a semi-quantitative culture and two PCR assays. If positive on fluidized undiluted specimen, the RDT was repeated on 1:100-diluted sample. The RDT was found highly specific when tested on non-S. pneumoniae strains. By comparison to culture and PCR assays, the RDT on undiluted secretions exhibited a sensitivity (Se) and negative predictive value (NPV) of more than 98%. By comparison to criteria of S. pneumoniae pneumonia combining typical symptoms, X-ray image, and culture ≥107 CFU/ml, the Se and NPV of RDT on diluted specimens were 100% in both cases.
Conclusions: In case of negative result, the excellent NPV of RDT on undiluted secretions allows excluding S. pneumoniae pneumonia. In case of positive result, the excellent sensitivity of RDT on diluted secretions for the diagnosis of S. pneumoniae pneumonia allows proposing a suitable antimicrobial treatment at day 0.
Keywords: Child; PCR assay; Pneumonia; Rapid diagnostic test; Respiratory infection; Streptococcus pneumoniae.
Conflict of interest statement
CHH is a PhD student of the University of Saint-Etienne whose thesis was cofounded by the CIFRE French Ministry of Higher Education and Research (CIFRE fellowship No 1283/2014) and BioSpeedia. EB and YG are working at BioSpeedia. The other authors have no conflict of interest to declare in relation with the matter of this study.
Figures

References
-
- Global Burden of Disease Child and Adolescent Health Collaboration. Kassebaum N, Kyu HH, et al. Child and adolescent health from 1990 to 2015: findings from the global burden of diseases, injuries, and risk factors 2015 Study. JAMA Pediatr. 2017;1716:573–592. doi: 10.1001/jamapediatrics.2017.0250. - DOI - PMC - PubMed
-
- Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(S2):1–23. - PubMed
-
- Freymuth F, Leven M, Wallet F. Lower respiratory tract infections. In: Cornaglia G, Courcol R, Herrmann JL, Kahlmeter G, Peigue-Lafeuille H, Vila J, SFM, ESCMID, editors. European manual of clinical microbiology. 1. France: Epernay; 2012. pp. 153–161.
-
- Botterel F, Cattoen C, Pozzetto B. Infections broncho-pulmonaires. REMIC Société Française de Microbiologie Ed. 2018. pp. 199–212.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

