Associated factors of poor treatment outcomes in patients with giant cell arteritis: clinical implication of large vessel lesions
- PMID: 32264967
- PMCID: PMC7137303
- DOI: 10.1186/s13075-020-02171-6
Associated factors of poor treatment outcomes in patients with giant cell arteritis: clinical implication of large vessel lesions
Abstract
Background: Relapses frequently occur in giant cell arteritis (GCA), and long-term glucocorticoid therapy is required. The identification of associated factors with poor treatment outcomes is important to decide the treatment algorithm of GCA.
Methods: We enrolled 139 newly diagnosed GCA patients treated with glucocorticoids between 2007 and 2014 in a retrospective, multi-center registry. Patients were diagnosed with temporal artery biopsy, 1990 American College of Rheumatology classification criteria, or large vessel lesions (LVLs) detected by imaging based on the modified classification criteria. Poor treatment outcomes (non-achievement of clinical remission by week 24 or relapse during 52 weeks) were evaluated. Clinical remission was defined as the absence of clinical signs and symptoms in cranial and large vessel areas, polymyalgia rheumatica (PMR), and elevation of C-reactive protein (CRP) levels. A patient was determined to have a relapse if he/she had either one of the signs and symptoms that newly appeared or worsened after achieving clinical remission. Re-elevation of CRP without clinical manifestations was considered as a relapse if other causes such as infection were excluded and the treatment was intensified. Associated factors with poor treatment outcomes were analyzed by using the Cox proportional hazard model.
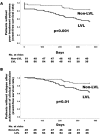
Results: Cranial lesions, PMR, and LVLs were detected in 77.7%, 41.7%, and 52.5% of the enrolled patients, respectively. Treatment outcomes were evaluated in 119 newly diagnosed patients who were observed for 24 weeks or longer. The mean initial dose of prednisolone was 0.76 mg/kg/day, and 29.4% received any concomitant immunosuppressive drugs at baseline. Overall, 41 (34.5%) of the 119 patients had poor treatment outcomes; 13 did not achieve clinical remission by week 24, and 28 had a relapse after achieving clinical remission. Cumulative rates of the events of poor treatment outcomes in patients with and without LVLs were 47.5% and 17.7%, respectively. A multivariable model showed the presence of LVLs at baseline was significantly associated with poor treatment outcomes (adjusted hazard ratio [HR] 3.54, 95% CI 1.52-8.24, p = 0.003). Cranial lesions and PMR did not increase the risk of poor treatment outcomes.
Conclusion: The initial treatment intensity in the treatment algorithm of GCA could be determined based upon the presence or absence of LVLs detected by imaging at baseline.
Keywords: Giant cell arteritis; Glucocorticoid therapy; Large vessel lesions; Poor treatment outcomes; Relapse; Remission.
Conflict of interest statement
TS has received research grants and/or honoraria from Abbvie Japan Co., Ltd., AsahiKASEI Co., Ltd.; Astellas Pharma Inc.; Ayumi Pharmaceutical; Bristol Myers Squibb K.K.; Chugai Pharmaceutical Co., Ltd.; Daiichi Sankyo; Eli Lilly Japan K.K.; Mitsubishi-Tanabe Pharma Co.; Ono Pharmaceutical; Pfizer Japan, Inc.; Takeda Pharmaceutical Co., Ltd.; and UCB Japan Co., Ltd. Tokyo Medical and Dental University received unrestricted research grants from Department of Lifetime Clinical Immunology from Ayumi Pharmaceutical Corporation; Chugai Pharmaceutical Co., Ltd.; CSL Behring K.K.; Japan Blood Products Organization; and UCB Japan Co., Ltd. HH has received honoraria from Chugai Pharmaceutical Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; Mitsubishi-Tanabe Pharma Co.; Astellas Pharma Inc.; Bristol Myers Squibb K.K.; Pfizer Japan Inc.; Eli Lilly Japan K.K.; AsahiKASEI Co., Ltd.; Teijin Pharma Ltd.; and Abbvie Japan Co., Ltd. HAU belongs to the Department of Chronic Kidney Disease and Cardiovascular Disease which is endowed by Chugai Pharmaceutical, MSD, Boehringer Ingelheim, and Kawanishi Holdings. HY has received lecture fees from Chugai Pharmaceutical Co., Ltd. and Nihon Medi-Physics Co., Ltd. YW have received honoraria from Chugai Pharmaceutical Co., Ltd. EA belongs to the Department of Therapeutic Strategy for Heart Failure, Graduate School of Medicine, University of Tokyo, which is endowed by Actelion Pharmaceuticals Japan Ltd., Otsuka Pharmaceutical, NIPRO CORPORATION, Terumo Corp., Senko Medical Instrument Mfg., Century Medical Inc., Kinetic Concepts Inc., and St. Jude Medical. EA has received honoraria from Takeda Pharmaceutical Co. Ltd., Bayer Yakuhin. Ltd., Otsuka Pharmaceutical Co., Ltd. YM has received honoraria from Chugai Pharmaceutical Co., Ltd. MK has nothing to declare. YM has received honoraria from Abbvie, Astellas, Ayumi Pharmaceutical, Bristol Myers Squibb, Chugai Pharmaceutical, Eisai Pharmaceutical, Janssen Pharmaceutical, Kissei Pharmaceutical, Nippon Kayaku, Pfizer Pharmaceutical, Takeda Pharmaceutical, and UCB Pharmaceutical and has received research grant support from Asahi Kasei Pharma, Chugai Pharmaceutical, Daiichi Sankyo, Eisai Pharmaceutical, Mitsubishi Tanabe Pharma, Nippon Kayaku, Ono Pharmaceutical, Gilead Sciences Inc., Janssen Pharmaceutical, and Teijin Pharma. NO and SF have nothing to declare. YK has received honoraria from Chugai Pharmaceutical Co., Ltd.; Glaxo-Smithkline K.K.; Sanofi K.K.; Pfizer Japan Inc.; and Asahi Kasei Pharma Corp. YK has received consulting fees from Chugai, Kyowa Hakko Kirin, Asahi Kasei, and UCB and speakers’ fees from Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Daiichi Sankyo, AbbVie, Nippon Shinyaku, and Towa. TN has received research grants and lecture fees from Chugai Pharmaceutical Co., Ltd. TO has received research grants from Chugai Pharmaceutical Co., Ltd.; Eisai Pharmaceutical.; and Actelion. YT has received consulting fees, speaking fees, and/or honoraria from Daiichi-Sankyo, Astellas, Pfizer, Mitsubishi-Tanabe, Bristol-Myers, Chugai, YL Biologics, Eli Lilly, Sanofi, Janssen, and UCB and has received research grants from Mitsubishi-Tanabe, Takeda, Bristol-Myers, Chugai, Astellas, Abbvie, MSD, Daiichi-Sankyo, Pfizer, Kyowa-Kirin, Eisai, and Ono. TT has received research grants from Astellas, Chugai, Daiichi Sankyo, Takeda, AbbVie, Asahi Kasei, Mitsubishi Tanabe, Pfizer, Eisai, AYUMI, Nippon Kayaku, and Novartis; has received consulting fees from Astra Zeneca, Eli Lilly, Novartis, Mitsubishi Tanabe, AbbVie, Nippon Kayaku, Janssen, Astellas, Taiho, Chugai, Taisho Toyama, GlaxoSmithKline, and UCB; and has served on speakers’ fees for AbbVie, Bristol-Myers Squibb, Chugai, Mitsubishi Tanabe, Pfizer, Astellas, Daiichi Sankyo, Eisai, Sanofi, Teijin, Takeda, and Novartis. YN has received research grants from Bayer Yakuhin, Ltd.; has received consulting fees and/or lecture fees and/or research grants from Chugai; has received consulting fees and/or lecture fees from AbbVie; and has received lecture fees from Astellas, Pfizer, MSD, Daiichi Sankyo, and Kowa. YA has received honoraria from Mitsubishi Tanabe Pharma Co.; Nippon Shinyaku Co., Ltd.; Chugai Pharmaceutical Co., Ltd.; Teijin Pharma, Ltd.; Asahi Kasei Corporation; AbbVie GK; F. Hoffmann-La Roche, Ltd.; GlaxoSmithKline K.K.; KYORIN Pharmaceutical Co., Ltd.; Kyowa Hakko Kirin Company, Ltd.; and Astellas Pharma Inc. MH has received research grants and/or honoraria from Abbott Japan Co., Ltd.; Astellas Pharma Inc.; Bristol-Myers Squibb K.K.; Chugai Pharmaceutical Co., Ltd.; Eisai Co., Ltd.; Janssen Pharmaceutical K.K.; Mitsubishi Tanabe Pharma Co.; Santen Pharmaceutical Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; Teijin Pharma, Ltd.; and Pfizer Japan Inc. MI has received research grants and/or honoraria from Chugai, Ono, Otsuka, Teijin, Mitsubishi-Tanabe, and Daiichi Sankyo.
Figures

References
-
- Kobayashi S, Yano T, Matsumoto Y, Numano F, Nakajima N, Yasuda K, et al. Clinical and epidemiologic analysis of giant cell (temporal) arteritis from a nationwide survey in 1998 in Japan: the first government-supported nationwide survey. Arthritis Rheum. 2003;49:594–598. doi: 10.1002/art.11195. - DOI - PubMed
-
- Nuenninghoff DM, Hunder GG, Christianson TJ, McClelland RL, Matteson EL. Mortality of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48:3532–3537. doi: 10.1002/art.11480. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

