Opportunistic infections in immunosuppressed patients with juvenile idiopathic arthritis: analysis by the Pharmachild Safety Adjudication Committee
- PMID: 32264969
- PMCID: PMC7136994
- DOI: 10.1186/s13075-020-02167-2
Opportunistic infections in immunosuppressed patients with juvenile idiopathic arthritis: analysis by the Pharmachild Safety Adjudication Committee
Abstract
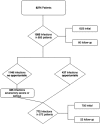
Background: To derive a list of opportunistic infections (OI) through the analysis of the juvenile idiopathic arthritis (JIA) patients in the Pharmachild registry by an independent Safety Adjudication Committee (SAC).
Methods: The SAC (3 pediatric rheumatologists and 2 pediatric infectious disease specialists) elaborated and approved by consensus a provisional list of OI for use in JIA. Through a 5 step-procedure, all the severe and serious infections, classified as per MedDRA dictionary and retrieved in the Pharmachild registry, were evaluated by the SAC by answering six questions and adjudicated with the agreement of 3/5 specialists. A final evidence-based list of OI resulted by matching the adjudicated infections with the provisional list of OI.
Results: A total of 772 infectious events in 572 eligible patients, of which 335 serious/severe/very severe non-OI and 437 OI (any intensity/severity), according to the provisional list, were retrieved. Six hundred eighty-two of 772 (88.3%) were adjudicated as infections, of them 603/682 (88.4%) as common and 119/682 (17.4%) as OI by the SAC. Matching these 119 opportunistic events with the provisional list, 106 were confirmed by the SAC as OI, and among them infections by herpes viruses were the most frequent (68%), followed by tuberculosis (27.4%). The remaining events were divided in the groups of non-OI and possible/patient and/or pathogen-related OI.
Conclusions: We found a significant number of OI in JIA patients on immunosuppressive therapy. The proposed list of OI, created by consensus and validated in the Pharmachild cohort, could facilitate comparison among future pharmacovigilance studies.
Trial registration: Clinicaltrials.gov NCT01399281; ENCePP seal: awarded on 25 November 2011.
Keywords: Biologics; Immunosuppressive therapy; Infections; Juvenile idiopathic arthritis; Opportunistic.
Conflict of interest statement
GG declares that she has no competing interests.
JFS has received sponsorship for a meeting by Sobi (< $10,000 USD).
EC declares that he has no competing interests.
AHG has received research grants from Gilead, Merck, Sharp & Dohme, and Pfizer; is or has been a consultant to Amplyx, Astellas, Basilea, F2G, Gilead, Merck, Sharp & Dohme, and Pfizer; and served at the speakers’ bureau of Astellas, Basilea, Gilead, Merck, Sharp & Dohme, Pfizer, and Schering-Plough. All of the above is < 10.000 per entity.
GH has received consultancies, speaking fees, and honoraria from Abbvie, Chugai, Pfizer, Novartis, Roche, and Sanofi (< $10,000 USD each).
HIH declares that he has no competing interests.
DJL has served on speaker bureaus for Genentech and Bristol-Meyers Squibb and served on a data and safety monitoring boards for Forest Research and the NIH-NIAMS; the Cincinnati Children’s Hospital Medical Center has received consulting fees for the work of Dr. Lovell from AbbVie, AstraZeneca, Bristol-Myers Squibb, Centocor, Genentech, Hoffman-La Roche, Lilly, Janssen, Novartis, Pfizer, Regeneron, R-Pharm and UBC. Each activity is less than $10,000.
TW declares that he has no competing interests.
TH declares that he has no competing interests.
PD has received speaker’s fees or consultancies or travel grants (all < 10,000 USD) from Medac, Novartis, Abbvie, Roche, SOBI, and Lilly.
HS declares that she has no competing interests.
GS has received honoraria as a sub-investigator in a Pfizer trial (> 10,000 USD).
FS declares that he has no competing interests.
DM declares that she has no competing interests.
TC has received consultancies, speaking fees, and honoraria from Roche and Abbvie < $10,000.
VV has received consultancies, speaking fees, and honoraria from Pfizer, Abbvie, and Sobi (< $10,000).
SS declares that she has no competing interests.
MR declares that she has no competing interests.
SKO has received teaching honoraria from Pfizer (< $10,000).
MC declares that he has no competing interests.
FBo received teaching honoraria from Novartis (< $10,000) and consulting fees from Biogen (< $10,000).
FBa declares that she has no competing interests.
AP declares that she has no competing interests.
AM: starting from 1 March 2016 to December 2018 Prof. Alberto Martini did not have any conflict of interest to declare since he was the Scientific Director of IRCCS Istituto Gaslini and this role did not allow him to render private consultancies resulting in personal income. He performed consultancy activities on behalf of the Gaslini Institute for the following companies: Abbvie, Biogen, Boehringer, Bristol-Myers and Squibb, EMD Serono, Janssen, Novartis, Pfizer, and R-Pharm. The money received for these activities was directly transferred to the Gaslini Institute’s bank account. Since January 2019, Prof. Alberto Martini is no longer the Scientific Director of IRCCS Istituto Gaslini; therefore, he can perform private consultancy services. Currently, he has active consultancy agreements with Janssen, Novartis, and Pfizer (< 10.000 USD each).
NW has received an institutional research grant from AbbVie (> 10,000 USD) for an e-health project. Consultancies: BMS (< 10,000 USD) on e-health developments.
NR has received honoraria for consultancies or speaker bureaus (< 10.000 USD each) from the following pharmaceutical companies in the past 3 years: Ablynx, AbbVie, Astrazeneca-Medimmune, Biogen, Boehringer, Bristol-Myers and Squibb, Eli-Lilly, EMD Serono, Glaxo Smith and Kline, Hoffmann-La Roche, Janssen, Merck, Novartis, Pfizer, R-Pharma, SanofiServier, Sinergie, Sobi, and Takeda. The Gaslini Hospital, where NR works as full-time public employee, has received contributions (> 10.000 USD each) from the following industries in the last 3 years: BMS, Eli-Lilly, GlaxoSmithKline, F Hoffmann-La Roche, Janssen, Novartis, Pfizer, and Sobi. This funding has been reinvested for the research activities of the hospital in a fully independent manner, without any commitment with third parties.
Figures
References
-
- Horneff G, Burgos-Vargas R, Constantin T, Foeldvari I, Vojinovic J, Chasnyk VG, et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann Rheum Dis. 2014;73(6):1114–1122. doi: 10.1136/annrheumdis-2012-203046. - DOI - PMC - PubMed
-
- Constantin T, Foeldvari I, Vojinovic J, Horneff G, Burgos-Vargas R, Nikishina I, et al. Two-year efficacy and safety of etanercept in pediatric patients with extended oligoarthritis, enthesitis-related arthritis, or psoriatic arthritis. J Rheumatol. 2016;43(4):816–824. doi: 10.3899/jrheum.150430. - DOI - PubMed