Arachidonate 12-lipoxygenase and 12-hydroxyeicosatetraenoic acid contribute to stromal aging-induced progression of pancreatic cancer
- PMID: 32265301
- PMCID: PMC7242692
- DOI: 10.1074/jbc.RA120.012798
Arachidonate 12-lipoxygenase and 12-hydroxyeicosatetraenoic acid contribute to stromal aging-induced progression of pancreatic cancer
Abstract
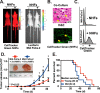
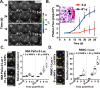
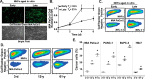
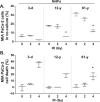
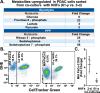
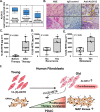
The incidence of pancreatic cancer increases with age, suggesting that chronological aging is a significant risk factor for this disease. Fibroblasts are the major nonmalignant cell type in the stroma of human pancreatic ductal adenocarcinoma (PDAC). In this study, we investigated whether the chronological aging of normal human fibroblasts (NHFs), a previously underappreciated area in pancreatic cancer research, influences the progression and therapeutic outcomes of PDAC. Results from experiments with murine xenografts and 2D and 3D co-cultures of NHFs and PDAC cells revealed that older NHFs stimulate proliferation of and confer resistance to radiation therapy of PDAC. MS-based metabolite analysis indicated that older NHFs have significantly increased arachidonic acid 12-lipoxygenase (ALOX12) expression and elevated levels of its mitogenic metabolite, 12-(S)-hydroxy-5,8,10,14-eicosatetraenoic acid (12-(S)-HETE) compared with their younger counterparts. In co-cultures with older rather than with younger NHFs, PDAC cells exhibited increases in mitogen-activated protein kinase signaling and cellular metabolism, as well as a lower oxidation state that correlated with their enhanced proliferation and resistance to radiation therapy. Expression of ALOX12 was found to be significantly lower in PDAC cell lines and tumor biopsies, suggesting that PDAC cells rely on a stromal supply of mitogens for their proliferative needs. Pharmacological (hydroxytyrosol) and molecular (siRNA) interventions of ALOX12 in older NHFs suppressed their ability to stimulate proliferation of PDAC cells. We conclude that chronological aging of NHFs contributes to PDAC progression and that ALOX12 and 12-(S)-HETE may be potential stromal targets for interventions that seek to halt progression and improve therapy outcomes.
Keywords: aging; arachidonic acid (AA) (ARA); cancer biology; cell proliferation; fibroblast; pancreatic cancer; stromal cell.
© 2020 Sarsour et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Yip D., Karapetis C., Strickland A., Steer C. B., and Goldstein D. (2006) Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst. Rev. 19, CD002093 - PubMed
-
- Tang D. G., Bhatia B., Tang S., and Schneider-Broussard R. (2007) 15-Lipoxygenase 2 (15-LOX2) is a functional tumor suppressor that regulates human prostate epithelial cell differentiation, senescence, and growth (size). Prostaglandins Other Lipid Mediat. 82, 135–146 10.1016/j.prostaglandins.2006.05.022 - DOI - PubMed
-
- Kadaba R., Birke H., Wang J., Hooper S., Andl C. D., Di Maggio F., Soylu E., Ghallab M., Bor D., Froeling F. E., Bhattacharya S., Rustgi A. K., Sahai E., Chelala C., Sasieni P., and Kocher H. M. (2013) Imbalance of desmoplastic stromal cell numbers drives aggressive cancer processes. J. Pathol. 230, 107–117 10.1002/path.4172 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

