Trehalose metabolism confers developmental robustness and stability in Drosophila by regulating glucose homeostasis
- PMID: 32265497
- PMCID: PMC7138798
- DOI: 10.1038/s42003-020-0889-1
Trehalose metabolism confers developmental robustness and stability in Drosophila by regulating glucose homeostasis
Abstract
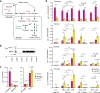
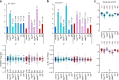
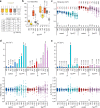
Organisms have evolved molecular mechanisms to ensure consistent and invariant phenotypes in the face of environmental fluctuations. Developmental homeostasis is determined by two factors: robustness, which buffers against environmental variations; and developmental stability, which buffers against intrinsic random variations. However, our understanding of these noise-buffering mechanisms remains incomplete. Here, we showed that appropriate glycemic control confers developmental homeostasis in the fruit fly Drosophila. We found that circulating glucose levels are buffered by trehalose metabolism, which acts as a glucose sink in circulation. Furthermore, mutations in trehalose synthesis enzyme (Tps1) increased the among-individual and within-individual variations in wing size. Whereas wild-type flies were largely resistant to changes in dietary carbohydrate and protein levels, Tps1 mutants experienced significant disruptions in developmental homeostasis in response to dietary stress. These results demonstrate that glucose homeostasis against dietary stress is crucial for developmental homeostasis.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Palmer AR, Strobeck C. Fluctuating asymmetry: measurement, analysis, patterns. Annu. Rev. Ecol. Syst. 1986;17:391–421. doi: 10.1146/annurev.es.17.110186.002135. - DOI
-
- Leamy LJL, Klingenberg CPK. The genetics and evolution of fluctuating asymmetry. Annu. Rev. Ecol. Evol. Syst. 2005;36:1–21. doi: 10.1146/annurev.ecolsys.36.102003.152640. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

