Nutrition Therapy, Glucose Control, and Brain Metabolism in Traumatic Brain Injury: A Multimodal Monitoring Approach
- PMID: 32265626
- PMCID: PMC7105880
- DOI: 10.3389/fnins.2020.00190
Nutrition Therapy, Glucose Control, and Brain Metabolism in Traumatic Brain Injury: A Multimodal Monitoring Approach
Abstract
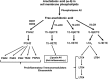
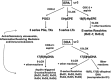
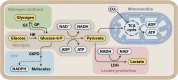
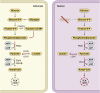
The goal of neurocritical care in patients with traumatic brain injury (TBI) is to prevent secondary brain damage. Pathophysiological mechanisms lead to loss of body mass, negative nitrogen balance, dysglycemia, and cerebral metabolic dysfunction. All of these complications have been shown to impact outcomes. Therapeutic options are available that prevent or mitigate their negative impact. Nutrition therapy, glucose control, and multimodality monitoring with cerebral microdialysis (CMD) can be applied as an integrated approach to optimize systemic immune and organ function as well as adequate substrate delivery to the brain. CMD allows real-time bedside monitoring of aspects of brain energy metabolism, by measuring specific metabolites in the extracellular fluid of brain tissue. Sequential monitoring of brain glucose and lactate/pyruvate ratio may reveal pathologic processes that lead to imbalances in supply and demand. Early recognition of these patterns may help individualize cerebral perfusion targets and systemic glucose control following TBI. In this direction, recent consensus statements have provided guidelines and recommendations for CMD applications in neurocritical care. In this review, we summarize data from clinical research on patients with severe TBI focused on a multimodal approach to evaluate aspects of nutrition therapy, such as timing and route; aspects of systemic glucose management, such as intensive vs. moderate control; and finally, aspects of cerebral metabolism. Research and clinical applications of CMD to better understand the interplay between substrate supply, glycemic variations, insulin therapy, and their effects on the brain metabolic profile were also reviewed. Novel mechanistic hypotheses in the interpretation of brain biomarkers were also discussed. Finally, we offer an integrated approach that includes nutritional and brain metabolic monitoring to manage severe TBI patients.
Keywords: brain glucose; cerebral microdialysis; glucose control; neurointensive care; nutrition therapy.
Copyright © 2020 Kurtz and Rocha.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

