Brain Metabolism Alterations in Type 2 Diabetes: What Did We Learn From Diet-Induced Diabetes Models?
- PMID: 32265637
- PMCID: PMC7101159
- DOI: 10.3389/fnins.2020.00229
Brain Metabolism Alterations in Type 2 Diabetes: What Did We Learn From Diet-Induced Diabetes Models?
Abstract
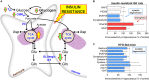
Type 2 diabetes (T2D) is a metabolic disease with impact on brain function through mechanisms that include glucose toxicity, vascular damage and blood-brain barrier (BBB) impairments, mitochondrial dysfunction, oxidative stress, brain insulin resistance, synaptic failure, neuroinflammation, and gliosis. Rodent models have been developed for investigating T2D, and have contributed to our understanding of mechanisms involved in T2D-induced brain dysfunction. Namely, mice or rats exposed to diabetogenic diets that are rich in fat and/or sugar have been widely used since they develop memory impairment, especially in tasks that depend on hippocampal processing. Here we summarize main findings on brain energy metabolism alterations underlying dysfunction of neuronal and glial cells promoted by diet-induced metabolic syndrome that progresses to a T2D phenotype.
Keywords: brain metabolism; diet-induced obesity; glucose; high-fat; insulin resistance; sucrose.
Copyright © 2020 Garcia-Serrano and Duarte.
Figures

References
-
- Baker L. D., Cross D. J., Minoshima S., Belongia D., Watson G. S., Craft S. (2011). Insulin resistance and alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol. 68 51–57. 10.1001/archneurol.2010.225 - DOI - PMC - PubMed
-
- Balakumar M., Raji L., Prabhu D., Sathishkumar C., Prabu P., Mohan V., et al. (2016). High-fructose diet is as detrimental as high-fat diet in the induction of insulin resistance and diabetes mediated by hepatic/pancreatic endoplasmic reticulum (ER) stress. Mol. Cell. Biochem. 423 93–104. 10.1007/s11010-016-2828-5 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

