Cycling Cross-Bridges Contribute to Thin Filament Activation in Human Slow-Twitch Fibers
- PMID: 32265723
- PMCID: PMC7105683
- DOI: 10.3389/fphys.2020.00144
Cycling Cross-Bridges Contribute to Thin Filament Activation in Human Slow-Twitch Fibers
Abstract
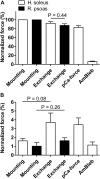
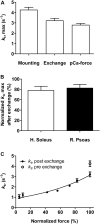
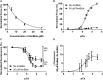
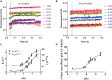
It has been shown that not only calcium but also strong binding myosin heads contribute to thin filament activation in isometrically contracting animal fast-twitch and cardiac muscle preparations. This behavior has not been studied in human muscle fibers or animal slow-twitch fibers. Human slow-twitch fibers are interesting since they contain the same myosin heavy chain isoform as the human heart. To explore myosin-induced activation of the thin filament in isometrically contracting human slow-twitch fibers, the endogenous troponin complex was exchanged for a well-characterized fast-twitch skeletal troponin complex labeled with the fluorescent dye N-((2-(Iodoacetoxy)ethyl)-N-methyl)amino-7-nitrobenz-2-oxa-1,3-diazole (fsTn-IANBD). The exchange was ≈70% complete (n = 8). The relative contributions of calcium and strong binding cross-bridges to thin filament activation were dissected by increasing the concentration of calcium from relaxing (pCa 7.5) to saturating levels (pCa 4.5) before and after incubating the exchanged fibers in the myosin inhibitor para-aminoblebbistatin (AmBleb). At pCa 4.5, the relative contributions of calcium and strong binding cross-bridges to thin filament activation were ≈69 and ≈31%, respectively. Additionally, switching from isometric to isotonic contraction at pCa 4.5 revealed that strong binding cross-bridges contributed ≈29% to thin filament activation (i.e., virtually the same magnitude obtained with AmBleb). Thus, we showed through two different approaches that lowering the number of strong binding cross-bridges, at saturating calcium, significantly reduced the activation of the thin filament in human slow-twitch fibers. The contribution of myosin to activation resembled that which was previously reported in rat cardiac and rabbit fast-twitch muscle preparations. This method could be applied to slow-twitch human fibers obtained from the soleus muscle of cardiomyopathy patients. Such studies could lead to a better understanding of the effect of point mutations of the cardiac myosin head on the regulation of muscle contraction and could lead to better management by pharmacological approaches.
Keywords: human striated muscle; myosin; regulation of muscle contraction; skinned fibers; slow-twitch muscle; thick filament; thin filament; troponin.
Copyright © 2020 López-Dávila, Chalovich, Zittrich, Piep, Matinmehr, Málnási-Csizmadia, Rauscher, Kraft, Brenner and Stehle.
Figures


Similar articles
-
Kinetics of thin filament activation probed by fluorescence of N-((2-(iodoacetoxy)ethyl)-N-methyl)amino-7-nitrobenz-2-oxa-1,3-diazole-labeled troponin I incorporated into skinned fibers of rabbit psoas muscle: implications for regulation of muscle contraction.Biophys J. 1999 Nov;77(5):2692-708. doi: 10.1016/S0006-3495(99)77103-1. Biophys J. 1999. PMID: 10545369 Free PMC article.
-
Thin filament activation probed by fluorescence of N-((2-(iodoacetoxy)ethyl)-N-methyl)amino-7-nitrobenz-2-oxa-1,3-diazole-labeled troponin I incorporated into skinned fibers of rabbit psoas muscle.Biophys J. 1999 Nov;77(5):2677-91. doi: 10.1016/S0006-3495(99)77102-X. Biophys J. 1999. PMID: 10545368 Free PMC article.
-
Myosin binding-induced cooperative activation of the thin filament in cardiac myocytes and skeletal muscle fibers.Biophys J. 1995 Apr;68(4):1430-42. doi: 10.1016/S0006-3495(95)80316-4. Biophys J. 1995. PMID: 7787029 Free PMC article.
-
Regulation of contraction in striated muscle.Physiol Rev. 2000 Apr;80(2):853-924. doi: 10.1152/physrev.2000.80.2.853. Physiol Rev. 2000. PMID: 10747208 Review.
-
Cross-bridges affect both TnC structure and calcium affinity in muscle fibers.Adv Exp Med Biol. 1993;332:183-92; discussion 192-4. doi: 10.1007/978-1-4615-2872-2_17. Adv Exp Med Biol. 1993. PMID: 8109332 Review.
Cited by
-
Assessing Cardiac Contractility From Single Molecules to Whole Hearts.JACC Basic Transl Sci. 2023 Oct 11;9(3):414-439. doi: 10.1016/j.jacbts.2023.07.013. eCollection 2024 Mar. JACC Basic Transl Sci. 2023. PMID: 38559627 Free PMC article. Review.
-
Cytotoxicity of snake venom Lys49 PLA2-like myotoxin on rat cardiomyocytes ex vivo does not involve a direct action on the contractile apparatus.Sci Rep. 2021 Sep 30;11(1):19452. doi: 10.1038/s41598-021-98594-5. Sci Rep. 2021. PMID: 34593882 Free PMC article.
-
Skeletal muscle fiber hypercontraction induced by Bothrops asper myotoxic phospholipases A2 ex vivo does not involve a direct action on the contractile apparatus.Pflugers Arch. 2023 Oct;475(10):1193-1202. doi: 10.1007/s00424-023-02840-w. Epub 2023 Jul 20. Pflugers Arch. 2023. PMID: 37474774 Free PMC article.
References
-
- Bell M. G., Lankford E. B., Gonye G. E., Ellis-Davies G. C., Martyn D. A., Regnier M., et al. (2006). Kinetics of cardiac thin-filament activation probed by fluorescence polarization of rhodamine-labeled troponin C in skinned guinea pig trabeculae. Biophys. J. 90 531–543. 10.1529/biophysj.105.072769 - DOI - PMC - PubMed
-
- Bremel R. D., Murray J. M., Weber A. (1973). Manifestations of cooperative behavior in regulated actin filament during actin-activated atp hydrolysis in presence of calcium. Cold Spring Harb. Symp. Quant. Biol. 37 267–274.
LinkOut - more resources
Full Text Sources

