In Human Autoimmunity, a Substantial Component of the B Cell Repertoire Consists of Polyclonal, Barely Mutated IgG+ve B Cells
- PMID: 32265907
- PMCID: PMC7099054
- DOI: 10.3389/fimmu.2020.00395
In Human Autoimmunity, a Substantial Component of the B Cell Repertoire Consists of Polyclonal, Barely Mutated IgG+ve B Cells
Abstract
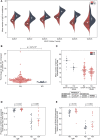
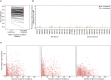
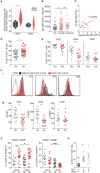
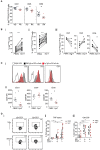
B cells are critical for promoting autoimmunity and the success of B cell depletion therapy in rheumatoid arthritis (RA) confirms their importance in driving chronic inflammation. Whilst disease specific autoantibodies are useful diagnostically, our understanding of the pathogenic B cell repertoire remains unclear. Defining it would lead to novel insights and curative treatments. To address this, we have undertaken the largest study to date of over 150 RA patients, utilizing next generation sequencing (NGS) to analyze up to 200,000 BCR sequences per patient. The full-length antigen-binding variable region of the heavy chain (IgGHV) of the IgG B cell receptor (BCR) were sequenced. Surprisingly, RA patients do not express particular clonal expansions of B cells at diagnosis. Rather they express a polyclonal IgG repertoire with a significant increase in BCRs that have barely mutated away from the germline sequence. This pattern remains even after commencing disease modifying therapy. These hypomutated BCRs are expressed by TNF-alpha secreting IgG+veCD27-ve B cells, that are expanded in RA peripheral blood and enriched in the rheumatoid synovium. A similar B cell repertoire is expressed by patients with Sjögren's syndrome. A rate limiting step in the initiation of autoimmunity is the activation of B cells and this data reveals that a sizeable component of the human autoimmune B cell repertoire consists of polyclonal, hypomutated IgG+ve B cells, that may play a critical role in driving chronic inflammation.
Keywords: B cells; BCR; TNF; autoimmunity; rheumatoid arthritis.
Copyright © 2020 Cowan, Miles, Capitani, Giguere, Johnsson, Goodyear, McInnes, Breusch, Gray and Gray.
Figures

References
-
- Porter D, van Melckebeke J, Dale J, Messow CM, McConnachie A, Walker A, et al. . Tumour necrosis factor inhibition versus rituximab for patients with rheumatoid arthritis who require biological treatment (ORBIT): an open-label, randomised controlled, non-inferiority, trial. Lancet. (2016) 388:239–47. 10.1016/S0140-6736(16)00380-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

