In vivo Effects of Romidepsin on T-Cell Activation, Apoptosis and Function in the BCN02 HIV-1 Kick&Kill Clinical Trial
- PMID: 32265913
- PMCID: PMC7100631
- DOI: 10.3389/fimmu.2020.00418
In vivo Effects of Romidepsin on T-Cell Activation, Apoptosis and Function in the BCN02 HIV-1 Kick&Kill Clinical Trial
Abstract
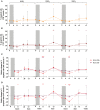
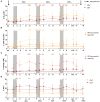
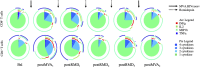
Romidepsin (RMD) is a well-characterized histone deacetylase inhibitor approved for the treatment of cutaneous T-cell lymphoma. in vitro and in vivo studies have demonstrated that it is able to induce HIV-1 gene expression in latently infected CD4+ T cells from HIV-1+ individuals on suppressive antiretroviral therapy. However, in vitro experiments suggested that RMD could also impair T-cell functionality, particularly of activated T cells. Thus, the usefulness of RMD in HIV-1 kick&kill strategies, that aim to enhance the immune system elimination of infected cells after inducing HIV-1 viral reactivation, may be limited. In order to address whether the in vitro observations are replicated in vivo, we determined the effects of RMD on the total and HIV-1-specific T-cell populations in longitudinal samples from the BCN02 kick&kill clinical trial (NCT02616874). BCN02 was a proof-of-concept study in 15 early treated HIV-1+ individuals that combined MVA.HIVconsv vaccination with three weekly infusions of RMD given as a latency reversing agent. Our results show that RMD induced a transient increase in the frequency of apoptotic T cells and an enhanced activation of vaccine-induced T cells. Although RMD reduced the number of vaccine-elicited T cells secreting multiple cytokines, viral suppressive capacity of CD8+ T cells was preserved over the RMD treatment. These observations have important implications for the design of effective kick&kill strategies for the HIV-1 cure.
Keywords: HDAC inhibitor; kick&kill strategy; latency reversing agent (LRA); romidepsin; therapeutic vaccine.
Copyright © 2020 Rosás-Umbert, Ruiz-Riol, Fernández, Marszalek, Coll, Manzardo, Cedeño, Miró, Clotet, Hanke, Moltó, Mothe, Brander and the BCN02 study group.
Figures

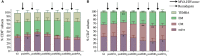

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

