Current understanding of metal ions in the pathogenesis of Alzheimer's disease
- PMID: 32266063
- PMCID: PMC7119290
- DOI: 10.1186/s40035-020-00189-z
Current understanding of metal ions in the pathogenesis of Alzheimer's disease
Abstract
Background: The homeostasis of metal ions, such as iron, copper, zinc and calcium, in the brain is crucial for maintaining normal physiological functions. Studies have shown that imbalance of these metal ions in the brain is closely related to the onset and progression of Alzheimer's disease (AD), the most common neurodegenerative disorder in the elderly.
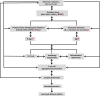
Main body: Erroneous deposition/distribution of the metal ions in different brain regions induces oxidative stress. The metal ions imbalance and oxidative stress together or independently promote amyloid-β (Aβ) overproduction by activating β- or γ-secretases and inhibiting α-secretase, it also causes tau hyperphosphorylation by activating protein kinases, such as glycogen synthase kinase-3β (GSK-3β), cyclin-dependent protein kinase-5 (CDK5), mitogen-activated protein kinases (MAPKs), etc., and inhibiting protein phosphatase 2A (PP2A). The metal ions imbalances can also directly or indirectly disrupt organelles, causing endoplasmic reticulum (ER) stress; mitochondrial and autophagic dysfunctions, which can cause or aggravate Aβ and tau aggregation/accumulation, and impair synaptic functions. Even worse, the metal ions imbalance-induced alterations can reversely exacerbate metal ions misdistribution and deposition. The vicious cycles between metal ions imbalances and Aβ/tau abnormalities will eventually lead to a chronic neurodegeneration and cognitive deficits, such as seen in AD patients.
Conclusion: The metal ions imbalance induces Aβ and tau pathologies by directly or indirectly affecting multiple cellular/subcellular pathways, and the disrupted homeostasis can reversely aggravate the abnormalities of metal ions transportation/deposition. Therefore, adjusting metal balance by supplementing or chelating the metal ions may be potential in ameliorating AD pathologies, which provides new research directions for AD treatment.
Keywords: Alzheimer’s disease; Amyloid-β; Autophagy; Metal ions; Oxidative stress; Synapses; Tau.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

