Mechanisms of Cardiovascular Disorders in Patients With Chronic Kidney Disease: A Process Related to Accelerated Senescence
- PMID: 32266265
- PMCID: PMC7099607
- DOI: 10.3389/fcell.2020.00185
Mechanisms of Cardiovascular Disorders in Patients With Chronic Kidney Disease: A Process Related to Accelerated Senescence
Abstract
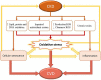
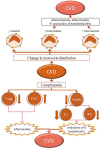
Cardiovascular diseases (CVDs), especially those involving a systemic inflammatory process such as atherosclerosis, remain the leading cause of morbidity and mortality in patients with chronic kidney disease (CKD). CKD is a systemic condition affecting approximately 10% of the general population. The prevalence of CKD has increased over the past decades because of the aging of the population worldwide. Indeed, CVDs in patients with CKD constitute a premature form of CVD observed in the general population. Multiple studies indicate that patients with renal disease undergo accelerated aging, which precipitates the appearance of pathologies, including CVDs, usually associated with advanced age. In this review, we discuss several aspects that characterize CKD-associated CVDs, such as etiopathogenic elements that CKD patients share with the general population, changes in the cellular balance of reactive oxygen species (ROS), and the associated process of cellular senescence. Uremia-associated aging is linked with numerous changes at the cellular and molecular level. These changes are similar to those observed in the normal process of physiologic aging. We also discuss new perspectives in the study of CKD-associated CVDs and epigenetic alterations in intercellular signaling, mediated by microRNAs and/or extracellular vesicles (EVs), which promote vascular damage and subsequent development of CVD. Understanding the processes and factors involved in accelerated senescence and other abnormal intercellular signaling will identify new therapeutic targets and lead to improved methods of diagnosis and monitoring for patients with CKD-associated CVDs.
Keywords: atherosclerosis; cardiovascular diseases; cellular senescence; chronic kidney disease; epigenetic alterations; extracellular vesicles; microRNAs; reactive oxygen species.
Copyright © 2020 Carracedo, Alique, Vida, Bodega, Ceprián, Morales, Praga, de Sequera and Ramírez.
Figures

References
-
- Annuk M., Zilmer M., Lind L., Linde T., Fellström B. (2001). Oxidative stress and endothelial function in chronic renal failure. J. Am. Soc. Nephrol. 12 2747–2752. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

