Nonclinical regulatory immunotoxicity testing of nanomedicinal products: Proposed strategy and possible pitfalls
- PMID: 32266791
- PMCID: PMC7507198
- DOI: 10.1002/wnan.1633
Nonclinical regulatory immunotoxicity testing of nanomedicinal products: Proposed strategy and possible pitfalls
Abstract
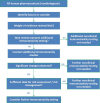
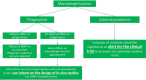
Various nanomedicinal products (NMPs) have been reported to induce an adverse immune response, which may be related to their tendency to accumulate in or target cells of the immune system. Therefore, before their market authorization, NMPs should be thoroughly evaluated for their immunotoxic potential. Nonclinical regulatory immunotoxicity testing of nonbiological medicinal products, including NMPs, is currently performed by following the guideline S8 "Immunotoxicity Studies for Human Pharmaceuticals" of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). However, this guideline does not cover all the immunotoxicity endpoints reported for NMPs in the literature, such as complement activation related pseudo allergy, hypersensitivity and immunosuppression. In addition, ICH-S8 does not provide any nanospecific testing considerations, which is important given their tendency to interfere with many commonly used toxicity assays. We therefore propose a nonclinical regulatory immunotoxicity assessment strategy, which considers the immunotoxicity endpoints currently missing in the ICH-S8. We also list the known pitfalls related to the testing of NMPs and how to tackle them. Next to defining the relevant physicochemical and pharmacokinetic properties of the NMP and its intended use, the proposed strategy includes an in vitro assay battery addressing various relevant immunotoxicity endpoints. A weight of evidence evaluation of this information can be used to shape the type and design of further in vivo investigations. The final outcome of the immunotoxicity assessment can be included in the overall risk assessment of the NMP and provide alerts for relevant endpoints to address during clinical investigation. This article is categorized under: Toxicology and Regulatory Issues in Nanomedicine > Regulatory and Policy Issues in Nanomedicine Toxicology and Regulatory Issues in Nanomedicine > Toxicology of Nanomaterials.
Keywords: ICH-S8; immunotoxicity; in vitro; nanomedicinal product; regulatory.
© 2020 The Authors. WIREs Nanomedicine and Nanobiotechnology published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors have declared no conflicts of interest for this article.
Figures



References
-
- Caron, W. P. , Lay, J. C. , Fong, A. M. , La‐Beck, N. M. , Kumar, P. , Newman, S. E. , … Zamboni, W. C. (2013). Translational studies of phenotypic probes for the mononuclear phagocyte system and liposomal pharmacology. The Journal of Pharmacology and Experimental Therapeutics, 347(3), 599–606. 10.1124/jpet.113.208801 - DOI - PMC - PubMed
-
- De Jong, W. H. , Van Der Ven, L. T. , Sleijffers, A. , Park, M. V. D. Z. , Jansen, E. H. , Van Loveren, H. , & Vandebriel, R. J. (2013). Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials, 34(33), 8333–8343. 10.1016/j.biomaterials.2013.06.048 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

