Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients
- PMID: 32267220
- PMCID: PMC7323511
- DOI: 10.3201/eid2607.200841
Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients
Abstract
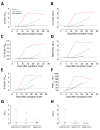
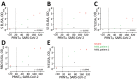
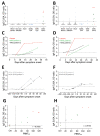
A new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has recently emerged to cause a human pandemic. Although molecular diagnostic tests were rapidly developed, serologic assays are still lacking, yet urgently needed. Validated serologic assays are needed for contact tracing, identifying the viral reservoir, and epidemiologic studies. We developed serologic assays for detection of SARS-CoV-2 neutralizing, spike protein-specific, and nucleocapsid-specific antibodies. Using serum samples from patients with PCR-confirmed SARS-CoV-2 infections, other coronaviruses, or other respiratory pathogenic infections, we validated and tested various antigens in different in-house and commercial ELISAs. We demonstrated that most PCR-confirmed SARS-CoV-2-infected persons seroconverted by 2 weeks after disease onset. We found that commercial S1 IgG or IgA ELISAs were of lower specificity, and sensitivity varied between the 2 assays; the IgA ELISA showed higher sensitivity. Overall, the validated assays described can be instrumental for detection of SARS-CoV-2-specific antibodies for diagnostic, seroepidemiologic, and vaccine evaluation studies.
Keywords: COVID-19; ELISA; HCoV; RBD; SARS-CoV-2; Severe acute respiratory syndrome coronavirus 2; antibodies; coronavirus; coronavirus disease 2019; human coronavirus; neutralization; nucleocapsid protein; receptor-binding domain; respiratory infections; serologic analysis; spike protein; viruses; zoonoses.
Figures


Comment in
-
The prevalence of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) IgG antibodies in intensive care unit (ICU) healthcare personnel (HCP) and its implications-a single-center, prospective, pilot study.Infect Control Hosp Epidemiol. 2021 May;42(5):638-639. doi: 10.1017/ice.2020.298. Epub 2020 Jun 12. Infect Control Hosp Epidemiol. 2021. PMID: 32530392 Free PMC article. No abstract available.
-
Review of Current Advances in Serologic Testing for COVID-19.Am J Clin Pathol. 2020 Aug 5;154(3):293-304. doi: 10.1093/ajcp/aqaa112. Am J Clin Pathol. 2020. PMID: 32583852 Free PMC article.
References
-
- World Health Organization. Coronavirus disease (COVID-2019) situation reports [cited 2020 Mar 14]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

