Efficacy and Safety of Continuous Risankizumab Therapy vs Treatment Withdrawal in Patients With Moderate to Severe Plaque Psoriasis: A Phase 3 Randomized Clinical Trial
- PMID: 32267471
- PMCID: PMC7142813
- DOI: 10.1001/jamadermatol.2020.0723
Efficacy and Safety of Continuous Risankizumab Therapy vs Treatment Withdrawal in Patients With Moderate to Severe Plaque Psoriasis: A Phase 3 Randomized Clinical Trial
Abstract
Importance: Risankizumab selectively inhibits interleukin 23, a cytokine that contributes to psoriatic inflammation.
Objective: To evaluate the efficacy and safety of risankizumab vs placebo and continuous treatment vs withdrawal in adults with moderate to severe plaque psoriasis.
Design, setting, and participants: Multinational, phase 3, randomized, double-blind, placebo-controlled trial conducted from March 6, 2016, to July 26, 2018. A total of 507 eligible patients had stable moderate to severe chronic plaque psoriasis for 6 months or longer, body surface area involvement greater than or equal to 10%, Psoriasis Area and Severity Index (PASI) greater than or equal to 12, and a static Physician's Global Assessment (sPGA) score greater than or equal to 3. Intention-to-treat analysis was conducted.
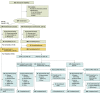
Interventions: Patients were randomized (4:1, interactive response technology) to risankizumab, 150 mg, subcutaneously, or placebo at weeks 0 and 4 (part A1). All patients received risankizumab at week 16. At week 28, patients randomized to risankizumab who achieved an sPGA score of 0/1 were rerandomized 1:2 to risankizumab or placebo every 12 weeks (part B).
Main outcomes and measures: Co-primary end points for the part A1 phase included proportions of patients achieving greater than or equal to 90% improvement in PASI (PASI 90) and sPGA score of 0/1 at week 16. The PASI measures severity of erythema, infiltration, and desquamation weighted by area of skin involvement over the head, trunk, upper extremities, and lower extremities; scores range from 0 (no disease) to 72 (maximal disease activity). The sPGA assesses average thickness, erythema, and scaling of all psoriatic lesions; scores range from 0 (clear) to 4 (severe), with 0/1 indicating clear or almost clear. Primary and secondary end points in part B included proportion of rerandomized patients achieving an sPGA score of 0/1 at week 52 (primary) and week 104 (secondary).
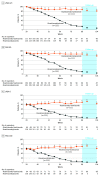
Results: Of 563 patients screened, 507 were randomized to risankizumab (n = 407) or placebo (n = 100). Most patients were men (356 [70.2%]); median age was 51 years (interquartile range, 38-60 years). At week 16, 298 patients (73.2%) in the treatment group vs 2 patients (2.0%) receiving placebo achieved a PASI 90 response, and 340 patients (83.5%) receiving risankizumab vs 7 patients (7.0%) receiving placebo achieved sPGA 0/1 scores (placebo-adjusted differences: PASI 90: 70.8%; 95% CI, 65.7%-76.0%; sPGA 0/1: 76.5%; 95% CI, 70.4%-82.5%; P < .001 for both). At week 28, 336 responders were rerandomized to risankizumab (n = 111) or treatment withdrawal (n = 225). At week 52, the sPGA 0/1 score was achieved by 97 patients (87.4%) receiving risankizumab vs 138 patients (61.3%) receiving placebo. At week 104, the sPGA 0/1 score was achieved by 90 patients (81.1%) receiving risankizumab vs 16 patients (7.1%) receiving placebo (placebo-adjusted differences: week 52: 25.9%; 95% CI, 17.3%-34.6%; week 104: 73.9%; 95% CI, 66.0%-81.9%; P < .001 for both). Rates of treatment-emergent adverse events were similar between risankizumab (186 [45.7%]) and placebo (49 [49.0%]) in part A1 and remained stable over time.
Conclusions and relevance: Risankizumab showed superior efficacy compared with placebo through 16 weeks and treatment withdrawal through 2 years. Risankizumab was well tolerated, with no unexpected safety findings during the 2-year trial.
Trial registration: ClinicalTrials.gov Identifier: NCT02672852.
Conflict of interest statement
Figures
References
-
- World Health Organization . Global Report on Psoriasis. World Health Organization; 2016.
-
- Vanaclocha F, Crespo-Erchiga V, Jiménez-Puya R, et al. ; Investigadores del estudio AQUILES . Immune-mediated inflammatory diseases and other comorbidities in patients with psoriasis: baseline characteristics of patients in the AQUILES study. Actas Dermosifiliogr. 2015;106(1):35-43. doi:10.1016/j.ad.2014.06.003 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

