Big Is Beautiful: Enhanced saRNA Delivery and Immunogenicity by a Higher Molecular Weight, Bioreducible, Cationic Polymer
- PMID: 32267667
- PMCID: PMC7304921
- DOI: 10.1021/acsnano.0c00326
Big Is Beautiful: Enhanced saRNA Delivery and Immunogenicity by a Higher Molecular Weight, Bioreducible, Cationic Polymer
Abstract
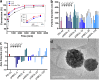
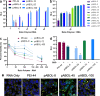
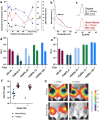
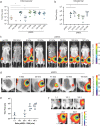
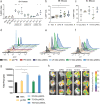
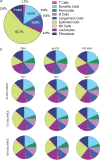
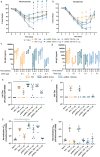
Self-amplifying RNA (saRNA) vaccines are highly advantageous, as they result in enhanced protein expression compared to mRNA (mRNA), thus minimizing the required dose. However, previous delivery strategies were optimized for siRNA or mRNA and do not necessarily deliver saRNA efficiently due to structural differences of these RNAs, thus motivating the development of saRNA delivery platforms. Here, we engineer a bioreducible, linear, cationic polymer called "pABOL" for saRNA delivery and show that increasing its molecular weight enhances delivery both in vitro and in vivo. We demonstrate that pABOL enhances protein expression and cellular uptake via both intramuscular and intradermal injection compared to commercially available polymers in vivo and that intramuscular injection confers complete protection against influenza challenge. Due to the scalability of polymer synthesis and ease of formulation preparation, we anticipate that this polymer is highly clinically translatable as a delivery vehicle for saRNA for both vaccines and therapeutics.
Keywords: RNA; influenza; nucleic acid; polymer; replicon; vaccine.
Conflict of interest statement
The authors declare the following competing financial interest(s): A.K.B., Y.Z., R.J.S., and M.M.S. are co-inventors on a patent resulting from this work.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

