Diamond-Graphene Composite Nanostructures
- PMID: 32267704
- PMCID: PMC7227005
- DOI: 10.1021/acs.nanolett.0c00556
Diamond-Graphene Composite Nanostructures
Abstract
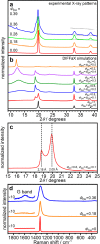
The search for new nanostructural topologies composed of elemental carbon is driven by technological opportunities as well as the need to understand the structure and evolution of carbon materials formed by planetary shock impact events and in laboratory syntheses. We describe two new families of diamond-graphene (diaphite) phases constructed from layered and bonded sp3 and sp2 nanostructural units and provide a framework for classifying the members of this new class of materials. The nanocomposite structures are identified within both natural impact diamonds and laboratory-shocked samples and possess diffraction features that have previously been assigned to lonsdaleite and postgraphite phases. The diaphite nanocomposites represent a new class of high-performance carbon materials that are predicted to combine the superhard qualities of diamond with high fracture toughness and ductility enabled by the graphitic units and the atomically defined interfaces between the sp3- and sp2-bonded nanodomains.
Keywords: Graphene-diamond nanocomposite; density functional theory calculations; high-resolution TEM; mechanical properties; sp2- and sp3-bonded nanomaterials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Hazen R. M.; Jones A. P.; Baross J. A.. Reviews in Mineralogy and Geochemistry. Carbon in Earth; Mineralogical Society of America, Geochemical Society: Washington, DC, 2013.
-
- Langenhorst F.; Campione M. Ideal and real structures of carbon forms with some remarks on the geological significance. J. Geol. Soc. 2019, 176, 337–347. 10.1144/jgs2018-056. - DOI
-
- Frondel C.; Marvin U. B. Lonsdaleite, a hexagonal polymorph of diamond. Nature 1967, 214, 587–589. 10.1038/214587a0. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

