A critical review on the potential impacts of neonicotinoid insecticide use: current knowledge of environmental fate, toxicity, and implications for human health
- PMID: 32267911
- PMCID: PMC11755762
- DOI: 10.1039/c9em00586b
A critical review on the potential impacts of neonicotinoid insecticide use: current knowledge of environmental fate, toxicity, and implications for human health
Abstract
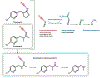
Neonicotinoid insecticides are widely used in both urban and agricultural settings around the world. Historically, neonicotinoid insecticides have been viewed as ideal replacements for more toxic compounds, like organophosphates, due in part to their perceived limited potential to affect the environment and human health. This critical review investigates the environmental fate and toxicity of neonicotinoids and their metabolites and the potential risks associated with exposure. Neonicotinoids are found to be ubiquitous in the environment, drinking water, and food, with low-level exposure commonly documented below acceptable daily intake standards. Available toxicological data from animal studies indicate possible genotoxicity, cytotoxicity, impaired immune function, and reduced growth and reproductive success at low concentrations, while limited data from ecological or cross-sectional epidemiological studies have identified acute and chronic health effects ranging from acute respiratory, cardiovascular, and neurological symptoms to oxidative genetic damage and birth defects. Due to the heavy use of neonicotinoids and potential for cumulative chronic exposure, these insecticides represent novel risks and necessitate further study to fully understand their risks to humans.
Conflict of interest statement
Conflicts of interest
There are no conflicts of interest to declare.
Figures
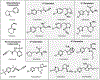
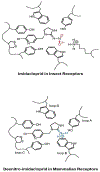
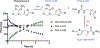

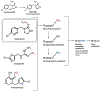
References
-
- Jeschke P, Nauen R, Schindler M. and Elbert A, Overview of the status and global strategy for neonicotinoids, Journal of Agriculture and Food Chemistry, 2010, 59, 2897–2908. - PubMed
-
- Myers C. and Hill E, Benefits of Neonicotinoid Seed Treatments to Soybean Production, U.S. Environmental Protection Agency, 2014.
-
- Tomizawa M. and Casida JE, Neonicotinoid insecticide toxicology: mechanisms of selective action, Annu. Rev. Pharmacol. Toxicol, 2005, 45, 247–268. - PubMed
-
- Hladik ML, Main AR and Goulson D, Environmental risks and challenges associated with neonicotinoid insecticides, Environ. Sci. Technol, 2018. - PubMed
-
- Jeschke P. and Nauen R, Neonicotinoids—from zero to hero in insecticide chemistry, Pest Manage. Sci, 2008, 64, 1084–1098. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

