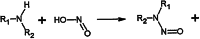The Finding of N-Nitrosodimethylamine in Common Medicines
- PMID: 32267983
- PMCID: PMC7288647
- DOI: 10.1634/theoncologist.2020-0142
The Finding of N-Nitrosodimethylamine in Common Medicines
Abstract
The FDA has ordered the withdrawal of all ranitidine products from the marketplace based on recent findings of increased and unacceptable levels of N‐nitrosodimethylamine (NDMA). This commentary reviews the sources and properties of NDMA, assesses the dangers it poses, and suggests measures to mitigate contamination.
Figures
References
-
- Scanlan RA. Formation and occurrence of nitrosamines in food. Cancer Res 1983;43(suppl 5):2435s–2440s. - PubMed
-
- Agency for Toxic Substance & Disease Registry . Public Health Statement for n‐Nitrosodimethylamine. Available at https://www.atsdr.cdc.gov/PHS/PHS.asp?id=882&tid=173. Accessed January 21, 2015.
-
- World Health Organization, International Agency for Research on Cancer . Some N‐nitroso‐compounds In: IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 17 Lyon, France, 1978.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources