Sterically Controlled C-H Olefination of Heteroarenes
- PMID: 32267990
- PMCID: PMC7384109
- DOI: 10.1002/anie.202004521
Sterically Controlled C-H Olefination of Heteroarenes
Abstract
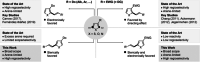
The regioselective functionalization of heteroarenes is a highly attractive synthetic target due to the prevalence of multiply substituted heteroarenes in nature and bioactive compounds. Some substitution patterns remain challenging: While highly efficient methods for the C2-selective olefination of 3-substituted five-membered heteroarenes have been reported, analogous methods to access the 5-olefinated products have remained limited by poor regioselectivities and/or the requirement to use an excess of the valuable heteroarene starting material. Herein we report a sterically controlled C-H olefination using heteroarenes as the limiting reagent. The method enables the highly C5-selective olefination of a wide range of heteroarenes and is shown to be useful in the context of late-stage functionalization.
Keywords: C−H activation; Fujiwara-Moritani reaction; heteroarenes; olefination; palladium.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

