Innate Immunity in the C. elegans Intestine Is Programmed by a Neuronal Regulator of AWC Olfactory Neuron Development
- PMID: 32268082
- PMCID: PMC7215899
- DOI: 10.1016/j.celrep.2020.03.042
Innate Immunity in the C. elegans Intestine Is Programmed by a Neuronal Regulator of AWC Olfactory Neuron Development
Abstract
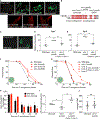
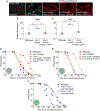
Olfactory neurons allow animals to discriminate nutritious food sources from potential pathogens. From a forward genetic screen, we uncovered a surprising requirement for the olfactory neuron gene olrn-1 in the regulation of intestinal epithelial immunity in Caenorhabditis elegans. During nematode development, olrn-1 is required to program the expression of odorant receptors in the AWC olfactory neuron pair. Here, we show that olrn-1 also functions in AWC neurons in the cell non-autonomous suppression of the canonical p38 MAPK PMK-1 immune pathway in the intestine. Low activity of OLRN-1, which activates the p38 MAPK signaling cassette in AWC neurons during larval development, also de-represses the p38 MAPK PMK-1 pathway in the intestine to promote immune effector transcription, increased clearance of an intestinal pathogen, and resistance to bacterial infection. These data reveal an unexpected connection between olfactory receptor development and innate immunity and show that anti-pathogen defenses in the intestine are developmentally programmed.
Keywords: AWC neurons; C. elegans; OLRN-1; immune homeostasis; intestinal immunity; olfactory neuron development; pathogen resistance.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests R.P.W. has submitted a patent application related to this work (U.S. patent application, 16/069,399, filed July 11, 2018). The authors declare no other potential competing interests.
Figures
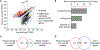


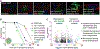
Similar articles
-
Left-right olfactory asymmetry results from antagonistic functions of voltage-activated calcium channels and the Raw repeat protein OLRN-1 in C. elegans.Neural Dev. 2007 Nov 6;2:24. doi: 10.1186/1749-8104-2-24. Neural Dev. 2007. PMID: 17986337 Free PMC article.
-
Aberrant Activation of p38 MAP Kinase-Dependent Innate Immune Responses Is Toxic to Caenorhabditis elegans.G3 (Bethesda). 2016 Jan 27;6(3):541-9. doi: 10.1534/g3.115.025650. G3 (Bethesda). 2016. PMID: 26818074 Free PMC article.
-
Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans.PLoS Pathog. 2011 Dec;7(12):e1002453. doi: 10.1371/journal.ppat.1002453. Epub 2011 Dec 22. PLoS Pathog. 2011. PMID: 22216003 Free PMC article.
-
Context Specificity of Stress-activated Mitogen-activated Protein (MAP) Kinase Signaling: The Story as Told by Caenorhabditis elegans.J Biol Chem. 2016 Apr 8;291(15):7796-804. doi: 10.1074/jbc.R115.711101. Epub 2016 Feb 23. J Biol Chem. 2016. PMID: 26907690 Free PMC article. Review.
-
Insights from the worm: the C. elegans model for innate immunity.Semin Immunol. 2014 Aug;26(4):303-9. doi: 10.1016/j.smim.2014.04.005. Epub 2014 May 21. Semin Immunol. 2014. PMID: 24856329 Free PMC article. Review.
Cited by
-
Innate immunity in C. elegans.Curr Top Dev Biol. 2021;144:309-351. doi: 10.1016/bs.ctdb.2020.12.007. Epub 2021 Mar 4. Curr Top Dev Biol. 2021. PMID: 33992157 Free PMC article. Review.
-
Non-canonical pattern recognition of a pathogen-derived metabolite by a nuclear hormone receptor identifies virulent bacteria in C. elegans.Immunity. 2023 Apr 11;56(4):768-782.e9. doi: 10.1016/j.immuni.2023.01.027. Epub 2023 Feb 17. Immunity. 2023. PMID: 36804958 Free PMC article.
-
Transcriptional suppression of sphingolipid catabolism controls pathogen resistance in C. elegans.PLoS Pathog. 2023 Oct 31;19(10):e1011730. doi: 10.1371/journal.ppat.1011730. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37906605 Free PMC article.
-
HLH-30/TFEB mediates sexual dimorphism in immunity in Caenorhabditis elegans.Autophagy. 2025 Feb;21(2):283-297. doi: 10.1080/15548627.2024.2375779. Epub 2024 Jul 20. Autophagy. 2025. PMID: 38963038 Free PMC article.
-
Opposing action of the FLR-2 glycoprotein hormone and DRL-1/FLR-4 MAP kinases balance p38-mediated growth and lipid homeostasis in C. elegans.PLoS Biol. 2023 Sep 29;21(9):e3002320. doi: 10.1371/journal.pbio.3002320. eCollection 2023 Sep. PLoS Biol. 2023. PMID: 37773960 Free PMC article.
References
-
- Bargmann CI, Hartwieg E, and Horvitz HR (1993). Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515–527. - PubMed
-
- Bauer Huang SL, Saheki Y, VanHoven MK, Torayama I, Ishihara T, Katsura I, van der Linden A, Sengupta P, and Bargmann CI (2007). Left-right olfactory asymmetry results from antagonistic functions of voltage-activated calcium channels and the Raw repeat protein OLRN-1 in C. elegans. Neural Dev 2, 24. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

