Treatment of Advanced Melanoma in 2020 and Beyond
- PMID: 32268150
- PMCID: PMC7541692
- DOI: 10.1016/j.jid.2020.03.943
Treatment of Advanced Melanoma in 2020 and Beyond
Abstract
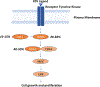
The melanoma field has seen an unprecedented set of clinical advances over the past decade. Therapeutic efficacy for advanced or metastatic melanoma went from being one of the most poorly responsive to one of the more responsive. Perhaps most strikingly, the advances that transformed management of the disease are based upon modern mechanism-based therapeutic strategies. The targeted approaches that primarily suppress the BRAF oncoprotein pathway have a high predictability of efficacy although less optimal depth or durability of response. Immunotherapy is primarily based on blockade of one or two immune checkpoints and has a lower predictability of response but higher fractions of durable remissions. This article reviews the clinical progress in management of advanced melanoma and also discusses the impact of the same therapies on earlier stage disease, where the agents have shown significant promise in treating resectable but high-risk clinical scenarios. Collectively, the progress in melanoma therapeutics has transformed the standard of care for patients, informed new approaches that are increasingly utilized for treatment of other malignancies, and suggest novel strategies to further boost efficacy for the many patients not yet receiving optimal benefit from these approaches.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement:
RWJ - Advisory Board: XSphera Biosciences. Research support: Monopteros Therapeutics
DEF- Board of Directors and Consultant: Soltego Inc
DEF has a financial interest in Soltego, Inc., a company developing SIK inhibitors for topical skin darkening treatments that might be used for a broad set of human applications. Dr. Fisher’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies.
RWJ has a financial interest in XSphera Biosciences Inc., a company focused on using ex vivo profiling technology to deliver functional, precision immune-oncology solutions for patients, providers, and drug development companies. RWJ’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Figures

Comment in
-
The Melanoma JID Connector Collection: An Introduction.J Invest Dermatol. 2020 Sep;140(9):1690. doi: 10.1016/j.jid.2020.04.004. J Invest Dermatol. 2020. PMID: 32800182 No abstract available.
References
-
- Arance HG AM, Dreno B, Flaherty KT, Demidov L, Stroyakovskiy D, Eroglu Z, Ferrucci PF, Pigozzo J, Rutkowski P, Mackiewicz J, Rooney I, Voulgari A, Troutman S, Pitcher B, Yan Y, Larkin JMG. COMBINATION TREATMENT WITH COBIMETINIB (C) AND ATEZOLIZUMAB (A) VS PEMBROLIZUMAB (P) IN PREVIOUSLY UNTREATED PATIENTS (PTS) WITH BRAFV600 WILD TYPE (WT) ADVANCED MELANOMA: PRIMARY ANALYSIS FROM THE PHASE 3 IMSPIRE170 TRIAL, https://oncologypro.esmo.org/meeting-resources/esmo-2019-congress/Combin...; 2019. [accessed 30 (suppl_5): v851–v934. 10.1093/annonc/mdz394]. - DOI
-
- Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol 2015;33(25):2780–8. - PubMed
-
- Andtbacka RHI, Collichio F, Harrington KJ, Middleton MR, Downey G, hrling K, et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J Immunother Cancer 2019;7(1):145. - PMC - PubMed
-
- Ascierto PA, Ferrucci PF, Fisher R, Del Vecchio M, Atkinson V, Schmidt H, et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med 2019;25(6):941–6. - PubMed
-
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17(7):2105–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

