Roles of Sorcin in Drug Resistance in Cancer: One Protein, Many Mechanisms, for a Novel Potential Anticancer Drug Target
- PMID: 32268494
- PMCID: PMC7226229
- DOI: 10.3390/cancers12040887
Roles of Sorcin in Drug Resistance in Cancer: One Protein, Many Mechanisms, for a Novel Potential Anticancer Drug Target
Abstract
The development of drug resistance is one of the main causes of failure in anti-cancer treatments. Tumor cells adopt many strategies to counteract the action of chemotherapeutic agents, e.g., enhanced DNA damage repair, inactivation of apoptotic pathways, alteration of drug targets, drug inactivation, and overexpression of ABC (Adenosine triphosphate-binding cassette, or ATP-binding cassette) transporters. These are broad substrate-specificity ATP-dependent efflux pumps able to export toxins or drugs out of cells; for instance, ABCB1 (MDR1, or P-glycoprotein 1), overexpressed in most cancer cells, confers them multidrug resistance (MDR). The gene coding for sorcin (SOluble Resistance-related Calcium-binding proteIN) is highly conserved among mammals and is located in the same chromosomal locus and amplicon as the ABC transporters ABCB1 and ABCB4, both in human and rodent genomes (two variants of ABCB1, i.e., ABCB1a and ABCB1b, are in rodent amplicon). Sorcin was initially characterized as a soluble protein overexpressed in multidrug (MD) resistant cells and named "resistance-related" because of its co-amplification with ABCB1. Although for years sorcin overexpression was thought to be only a by-product of the co-amplification with ABC transporter genes, many papers have recently demonstrated that sorcin plays an important part in MDR, indicating a possible role of sorcin as an oncoprotein. The present review illustrates sorcin roles in the generation of MDR via many mechanisms and points to sorcin as a novel potential target of different anticancer molecules.
Keywords: ABCB1; calcium; cancers; chemotherapeutic drugs; endoplasmic reticulum; multidrug resistance; sorcin.
Conflict of interest statement
The authors display no conflict of interest.
Figures



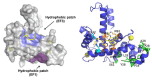


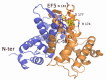
References
-
- Genovese I., Ilari A., Battista T., Chiarini F., Fiorillo A., Colotti G. Molecular bases of Sorcin-dependent resistance to chemotherapeutic agents. Cancer Drug Resist. 2018;1:17. doi: 10.20517/cdr.2018.10. - DOI
-
- Zamparelli C., Ilari A., Verzili D., Giangiacomo L., Colotti G., Pascarella S., Chiancone E. Structure-function relationships in sorcin, a member of the penta EF-hand family. Interaction of sorcin fragments with the ryanodine receptor and an Escherichia coli model system. Biochemistry. 2000;39:658–666. doi: 10.1021/bi991648v. - DOI - PubMed
-
- Colotti G., Zamparelli C., Verzili D., Mella M., Loughrey C.M., Smith G.L., Chiancone E. The W105G and W99G sorcin mutants demonstrate the role of the D helix in the Ca(2+)-dependent interaction with annexin VII and the cardiac ryanodine receptor. Biochemistry. 2006;45:12519–12529. doi: 10.1021/bi060416a. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

