Trophectoderm-Specific Knockdown of LIN28 Decreases Expression of Genes Necessary for Cell Proliferation and Reduces Elongation of Sheep Conceptus
- PMID: 32268593
- PMCID: PMC7177537
- DOI: 10.3390/ijms21072549
Trophectoderm-Specific Knockdown of LIN28 Decreases Expression of Genes Necessary for Cell Proliferation and Reduces Elongation of Sheep Conceptus
Abstract
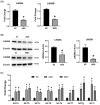
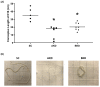
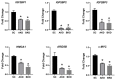
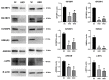
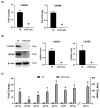
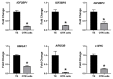
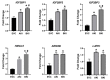
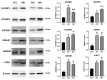
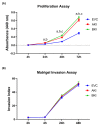
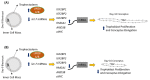
LIN28 inhibits let-7 miRNA maturation which prevents cell differentiation and promotes proliferation. We hypothesized that the LIN28-let-7 axis regulates proliferation-associated genes in sheep trophectoderm in vivo. Day 9-hatched sheep blastocysts were incubated with lentiviral particles to deliver shRNA targeting LIN28 specifically to trophectoderm cells. At day 16, conceptus elongation was significantly reduced in LIN28A and LIN28B knockdowns. Let-7 miRNAs were significantly increased and IGF2BP1-3, HMGA1, ARID3B, and c-MYC were decreased in trophectoderm from knockdown conceptuses. Ovine trophoblast (OTR) cells derived from day 16 trophectoderm are a useful tool for in vitro experiments. Surprisingly, LIN28 was significantly reduced and let-7 miRNAs increased after only a few passages of OTR cells, suggesting these passaged cells represent a more differentiated phenotype. To create an OTR cell line more similar to day 16 trophectoderm we overexpressed LIN28A and LIN28B, which significantly decreased let-7 miRNAs and increased IGF2BP1-3, HMGA1, ARID3B, and c-MYC compared to control. This is the first study showing the role of the LIN28-let-7 axis in trophoblast proliferation and conceptus elongation in vivo. These results suggest that reduced LIN28 during early placental development can lead to reduced trophoblast proliferation and sheep conceptus elongation at a critical period for successful establishment of pregnancy.
Keywords: cell proliferation; gene regulation; let-7 miRNAs; placenta; trophectoderm.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

