Neonatal Stroke and TLR1/2 Ligand Recruit Myeloid Cells through the Choroid Plexus in a CX3CR1-CCR2- and Context-Specific Manner
- PMID: 32269105
- PMCID: PMC7204080
- DOI: 10.1523/JNEUROSCI.2149-19.2020
Neonatal Stroke and TLR1/2 Ligand Recruit Myeloid Cells through the Choroid Plexus in a CX3CR1-CCR2- and Context-Specific Manner
Abstract
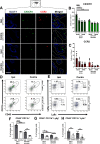
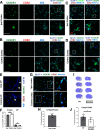
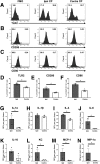
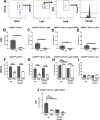
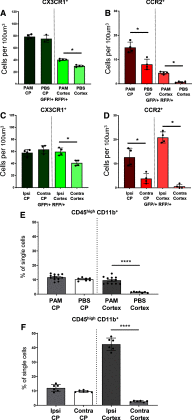
Neonatal stroke is as frequent as stroke in the elderly, but many pathophysiological injury aspects are distinct in neonates, including immune signaling. While myeloid cells can traffic into the brain via multiple routes, the choroid plexus (CP) has been identified as a uniquely educated gate for immune cell traffic during health and disease. To understand the mechanisms of myeloid cell trafficking via the CP and their influence on neonatal stroke, we characterized the phenotypes of CP-infiltrating myeloid cells after transient middle cerebral artery occlusion (tMCAO) in neonatal mice of both sexes in relation to blood-brain barrier permeability, injury, microglial activation, and CX3CR1-CCR2 signaling, focusing on the dynamics early after reperfusion. We demonstrate rapid recruitment of multiple myeloid phenotypes in the CP ipsilateral to the injury, including inflammatory CD45+CD11b+Ly6chighCD86+, beneficial CD45+CD11b+Ly6clowCD206+, and CD45+CD11b+Ly6clowLy6ghigh cells, but only minor leukocyte infiltration into acutely ischemic-reperfused cortex and negligible vascular albumin leakage. We report that CX3CR1-CCR2-mediated myeloid cell recruitment contributes to stroke injury. Considering the complexity of inflammatory cascades triggered by stroke and a role for TLR2 in injury, we also used direct TLR2 stimulation as an independent injury model. TLR2 agonist rapidly recruited myeloid cells to the CP, increased leukocytosis in the CSF and blood, but infiltration into the cortex remained low over time. While the magnitude and the phenotypes of myeloid cells diverged between tMCAO and TLR2 stimulation, in both models, disruption of CX3CR1-CCR2 signaling attenuated both monocyte and neutrophil trafficking to the CP and cortex.SIGNIFICANCE STATEMENT Stroke during the neonatal period leads to long-term disabilities. The mechanisms of ischemic injury and inflammatory response differ greatly between the immature and adult brain. We examined leukocyte trafficking via the choroid plexus (CP) following neonatal stroke in relation to blood-brain barrier integrity, injury, microglial activation, and signaling via CX3CR1 and CCR2 receptors, or following direct TLR2 stimulation. Ischemia-reperfusion triggered marked unilateral CX3CR1-CCR2 dependent accumulation of diverse leukocyte subpopulations in the CP without inducing extravascular albumin leakage or major leukocyte infiltration into the brain. Disrupted CX3CR1-CCR2 signaling was neuroprotective in part by attenuating monocyte and neutrophil trafficking. Understanding the migratory patterns of CP-infiltrating myeloid cells with intact and disrupted CX3CR1-CCR2 signaling could identify novel therapeutic targets to protect the neonatal brain.
Keywords: immune; inflammation; ischemia; microglia; perinatal.
Copyright © 2020 the authors.
Figures




Similar articles
-
CX3CR1-CCR2-dependent monocyte-microglial signaling modulates neurovascular leakage and acute injury in a mouse model of childhood stroke.J Cereb Blood Flow Metab. 2019 Oct;39(10):1919-1935. doi: 10.1177/0271678X18817663. Epub 2019 Jan 10. J Cereb Blood Flow Metab. 2019. PMID: 30628839 Free PMC article.
-
Scavenger receptor CD36 governs recruitment of myeloid cells to the blood-CSF barrier after stroke in neonatal mice.J Neuroinflammation. 2022 Feb 11;19(1):47. doi: 10.1186/s12974-022-02388-z. J Neuroinflammation. 2022. PMID: 35148760 Free PMC article.
-
Spatio-temporal profile, phenotypic diversity, and fate of recruited monocytes into the post-ischemic brain.J Neuroinflammation. 2016 Nov 4;13(1):285. doi: 10.1186/s12974-016-0750-0. J Neuroinflammation. 2016. PMID: 27814740 Free PMC article.
-
Cellular players that shape evolving pathology and neurodegeneration following traumatic brain injury.Brain Behav Immun. 2018 Jul;71:9-17. doi: 10.1016/j.bbi.2018.03.033. Epub 2018 Mar 27. Brain Behav Immun. 2018. PMID: 29601944 Review.
-
Tickets to the brain: role of CCR2 and CX3CR1 in myeloid cell entry in the CNS.J Neuroimmunol. 2010 Jul 27;224(1-2):80-4. doi: 10.1016/j.jneuroim.2010.05.015. Epub 2010 May 31. J Neuroimmunol. 2010. PMID: 20554025 Review.
Cited by
-
Peripheral immune cells and perinatal brain injury: a double-edged sword?Pediatr Res. 2022 Jan;91(2):392-403. doi: 10.1038/s41390-021-01818-7. Epub 2021 Nov 8. Pediatr Res. 2022. PMID: 34750522 Free PMC article. Review.
-
Structural and Functional Remodeling of the Brain Vasculature Following Stroke.Front Physiol. 2020 Aug 7;11:948. doi: 10.3389/fphys.2020.00948. eCollection 2020. Front Physiol. 2020. PMID: 32848875 Free PMC article. Review.
-
Prenatal administration of multipotent adult progenitor cells modulates the systemic and cerebral immune response in an ovine model of chorioamnionitis.Brain Behav Immun Health. 2022 May 2;23:100458. doi: 10.1016/j.bbih.2022.100458. eCollection 2022 Aug. Brain Behav Immun Health. 2022. PMID: 35647567 Free PMC article.
-
Targeting CX3CR1 Signaling Dynamics: A Critical Determinant in the Temporal Regulation of Post-Stroke Neurorepair.Brain Sci. 2025 Jul 17;15(7):759. doi: 10.3390/brainsci15070759. Brain Sci. 2025. PMID: 40722349 Free PMC article. Review.
-
Choroid Plexus Aquaporins in CSF Homeostasis and the Glymphatic System: Their Relevance for Alzheimer's Disease.Int J Mol Sci. 2023 Jan 3;24(1):878. doi: 10.3390/ijms24010878. Int J Mol Sci. 2023. PMID: 36614315 Free PMC article. Review.
References
-
- Andrade EB, Alves J, Madureira P, Oliveira L, Ribeiro A, Cordeiro-da-Silva A, Correia-Neves M, Trieu-Cuot P, Ferreira P (2013) TLR2-induced IL-10 production impairs neutrophil recruitment to infected tissues during neonatal bacterial sepsis. J Immunol 191:4759–4768. 10.4049/jimmunol.1301752 - DOI - PubMed
-
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA (2016) New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci USA 113:E1738–E1746. 10.1073/pnas.1525528113 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
