Venlafaxine Stimulates an MMP-9-Dependent Increase in Excitatory/Inhibitory Balance in a Stress Model of Depression
- PMID: 32269106
- PMCID: PMC7252486
- DOI: 10.1523/JNEUROSCI.2387-19.2020
Venlafaxine Stimulates an MMP-9-Dependent Increase in Excitatory/Inhibitory Balance in a Stress Model of Depression
Abstract
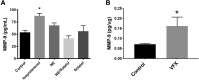
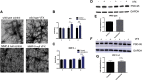
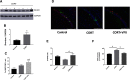
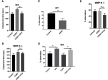
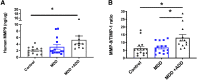
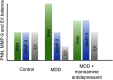
Emerging evidence suggests that there is a reduction in overall cortical excitatory to inhibitory balance in major depressive disorder (MDD), which afflicts ∼14%-20% of individuals. Reduced pyramidal cell arborization occurs with stress and MDD, and may diminish excitatory neurotransmission. Enhanced deposition of perineuronal net (PNN) components also occurs with stress. Since parvalbumin-expressing interneurons are the predominant cell population that is enveloped by PNNs, which enhance their ability to release GABA, excess PNN deposition likely increases pyramidal cell inhibition. In the present study, we investigate the potential for matrix metalloprotease-9 (MMP-9), an endopeptidase secreted in response to neuronal activity, to contribute to the antidepressant efficacy of the serotonin/norepinephrine reuptake inhibitor venlafaxine in male mice. Chronic venlafaxine increases MMP-9 levels in murine cortex, and increases both pyramidal cell arborization and PSD-95 expression in the cortex of WT but not MMP-9-null mice. We have previously shown that venlafaxine reduces PNN deposition and increases the power of ex vivo γ oscillations in conventionally housed mice. γ power is increased with pyramidal cell disinhibition and with remission from MDD. Herein we observe that PNN expression is increased in a corticosterone-induced stress model of disease and reduced by venlafaxine. Compared with mice that receive concurrent venlafaxine, corticosterone-treated mice also display reduced ex vivo γ power and impaired working memory. Autopsy-derived PFC samples show elevated MMP-9 levels in antidepressant-treated MDD patients compared with controls. These preclinical and postmortem findings highlight a link between extracellular matrix regulation and MDD.SIGNIFICANCE STATEMENT Reduced excitatory neurotransmission occurs with major depressive disorder, and may be normalized by antidepressant treatment. Underlying molecular mechanisms are, however, not well understood. Herein we investigate a potential role for an extracellular protease, released from neurons and known to play a role in learning and memory, in antidepressant-associated increases in excitatory transmission. Our data suggest that this protease, matrix metalloprotease-9, increases branching of excitatory neurons and concomitantly attenuates the perineuronal net to potentially reduce inhibitory input to these neurons. Matrix metalloprotease-9 may thus enhance overall excitatory/inhibitory balance and neuronal population dynamics, which are important to mood and memory.
Keywords: MMP; MMP-9; depression; parvalbumin; perineuronal net; venlafaxine.
Copyright © 2020 the authors.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
