Potential Role of Nonneutralizing IgA Antibodies in Cross-Protective Immunity against Influenza A Viruses of Multiple Hemagglutinin Subtypes
- PMID: 32269119
- PMCID: PMC7307104
- DOI: 10.1128/JVI.00408-20
Potential Role of Nonneutralizing IgA Antibodies in Cross-Protective Immunity against Influenza A Viruses of Multiple Hemagglutinin Subtypes
Abstract
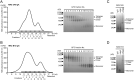
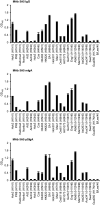
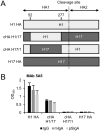
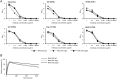
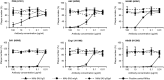
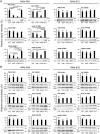
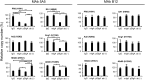
IgA antibodies on mucosal surfaces are known to play an important role in protection from influenza A virus (IAV) infection and are believed to be more potent than IgG for cross-protective immunity against IAVs of multiple hemagglutinin (HA) subtypes. However, in general, neutralizing antibodies specific to HA are principally HA subtype specific. Here, we focus on nonneutralizing but broadly cross-reactive HA-specific IgA antibodies. Recombinant IgG, monomeric IgA (mIgA), and polymeric secretory IgA (pSIgA) antibodies were generated based on the sequence of a mouse anti-HA monoclonal antibody (MAb) 5A5 that had no neutralizing activity but showed broad binding capacity to multiple HA subtypes. While confirming that there was no neutralizing activity of the recombinant MAbs against IAV strains A/Puerto Rico/8/1934 (H1N1), A/Adachi/2/1957 (H2N2), A/Hong Kong/483/1997 (H5N1), A/shearwater/South Australia/1/1972 (H6N5), A/duck/England/1/1956 (H11N6), and A/duck/Alberta/60/1976 (H12N5), we found that pSIgA, but not mIgA and IgG, significantly reduced budding and release of most of the viruses from infected cells. Electron microscopy demonstrated that pSIgA deposited newly produced virus particles on the surfaces of infected cells, most likely due to tethering of virus particles. Furthermore, we found that pSIgA showed significantly higher activity to reduce plaque sizes of the viruses than IgG and mIgA. These results suggest that nonneutralizing pSIgA reactive to multiple HA subtypes may play a role in intersubtype cross-protective immunity against IAVs.IMPORTANCE Mucosal immunity represented by pSIgA plays important roles in protection from IAV infection. Furthermore, IAV HA-specific pSIgA antibodies are thought to contribute to cross-protective immunity against multiple IAV subtypes. However, the mechanisms by which pSIgA exerts such versatile antiviral activity are not fully understood. In this study, we generated broadly cross-reactive recombinant IgG and pSIgA having the same antigen-recognition site and compared their antiviral activities in vitro These recombinant antibodies did not show "classical" neutralizing activity, whereas pSIgA, but not IgG, significantly inhibited the production of progeny virus particles from infected cells. Plaque formation was also significantly reduced by pSIgA, but not IgG. These effects were seen in infection with IAVs of several different HA subtypes. Based on our findings, we propose an antibody-mediated host defense mechanism by which mucosal immunity may contribute to broad cross-protection from IAVs of multiple HA subtypes, including viruses with pandemic potential.
Keywords: IgA; antibody; broadly cross-reactive; budding; cross-protective immunity; hemagglutinin; influenza A virus; nonneutralizing.
Copyright © 2020 American Society for Microbiology.
Figures



Similar articles
-
Heterosubtypic antiviral activity of hemagglutinin-specific antibodies induced by intranasal immunization with inactivated influenza viruses in mice.PLoS One. 2013 Aug 16;8(8):e71534. doi: 10.1371/journal.pone.0071534. eCollection 2013. PLoS One. 2013. PMID: 23977065 Free PMC article.
-
Comparison of antiviral activity between IgA and IgG specific to influenza virus hemagglutinin: increased potential of IgA for heterosubtypic immunity.PLoS One. 2014 Jan 17;9(1):e85582. doi: 10.1371/journal.pone.0085582. eCollection 2014. PLoS One. 2014. PMID: 24465606 Free PMC article.
-
A Novel Mechanism Underlying Antiviral Activity of an Influenza Virus M2-Specific Antibody.J Virol. 2020 Dec 9;95(1):e01277-20. doi: 10.1128/JVI.01277-20. Print 2020 Dec 9. J Virol. 2020. PMID: 33055251 Free PMC article.
-
One step closer to universal influenza epitopes.Expert Rev Anti Infect Ther. 2009 Aug;7(6):687-90. doi: 10.1586/eri.09.48. Expert Rev Anti Infect Ther. 2009. PMID: 19681695 Review.
-
Influenza Hemagglutinin Structures and Antibody Recognition.Cold Spring Harb Perspect Med. 2020 Aug 3;10(8):a038778. doi: 10.1101/cshperspect.a038778. Cold Spring Harb Perspect Med. 2020. PMID: 31871236 Free PMC article. Review.
Cited by
-
Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost immunization.Nat Commun. 2021 Nov 26;12(1):6871. doi: 10.1038/s41467-021-27063-4. Nat Commun. 2021. PMID: 34836955 Free PMC article.
-
A High-Yield Recombinant Inactivated Whole-Virion Nasal Influenza A(H1N1)pdm09 Virus Vaccine with an Attenuated PB2 Gene.Int J Mol Sci. 2025 Jun 7;26(12):5489. doi: 10.3390/ijms26125489. Int J Mol Sci. 2025. PMID: 40564953 Free PMC article.
-
Heterologous Systemic Prime-Intranasal Boosting Using a Spore SARS-CoV-2 Vaccine Confers Mucosal Immunity and Cross-Reactive Antibodies in Mice as well as Protection in Hamsters.Vaccines (Basel). 2022 Nov 10;10(11):1900. doi: 10.3390/vaccines10111900. Vaccines (Basel). 2022. PMID: 36366408 Free PMC article.
-
Heterologous prime-boost immunisation with mRNA- and AdC68-based 2019-nCoV variant vaccines induces broad-spectrum immune responses in mice.Front Immunol. 2023 Mar 15;14:1142394. doi: 10.3389/fimmu.2023.1142394. eCollection 2023. Front Immunol. 2023. PMID: 37006275 Free PMC article.
-
A mouse protozoan boosts antigen-specific mucosal IgA responses in a specific lipid metabolism- and signaling-dependent manner.Nat Commun. 2024 Sep 10;15(1):7914. doi: 10.1038/s41467-024-52336-z. Nat Commun. 2024. PMID: 39256385 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

