A Common Food Glycan, Pectin, Shares an Antigen with Streptococcus pneumoniae Capsule
- PMID: 32269150
- PMCID: PMC7142292
- DOI: 10.1128/mSphere.00074-20
A Common Food Glycan, Pectin, Shares an Antigen with Streptococcus pneumoniae Capsule
Erratum in
-
Erratum for Nahm et al., "A Common Food Glycan, Pectin, Shares an Antigen with Streptococcus pneumoniae Capsule".mSphere. 2020 Apr 29;5(2):e00363-20. doi: 10.1128/mSphere.00363-20. mSphere. 2020. PMID: 32350097 Free PMC article. No abstract available.
Abstract
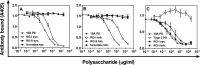
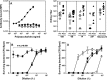
We are exposed daily to many glycans from bacteria and food plants. Bacterial glycans are generally antigenic and elicit antibody responses. It is unclear if food glycans' sharing of antigens with bacterial glycans influences our immune responses to bacteria. We studied 14 different plant foods for cross-reactivity with monoclonal antibodies (MAbs) against 24 pneumococcal serotypes which commonly cause infections and are included in pneumococcal vaccines. Serotype 15B-specific MAb cross-reacts with fruit peels, and serotype 10A MAb cross-reacts with many natural and processed plant foods. The serotype 10A cross-reactive epitope is terminal 1,6-linked β-galactose [βGal(1-6)], present in the rhamno-galacturonan I (RG-I) domain of pectin. Despite wide consumption of pectin, the immune response to 10A is comparable to the responses to other serotypes. An antipectin antibody can opsonize serotype 10A pneumococci, and the shared βGal(1-6) may be useful as a simple vaccine against 10A. Impact of food glycans should be considered in host-pathogen interactions and future vaccine designs.IMPORTANCE The impact of food consumption on vaccine responses is unknown. Streptococcus pneumoniae (the pneumococcus) is an important human pathogen, and its polysaccharide capsule is used as a vaccine. We show that capsule type 10A in a pneumococcal vaccine shares an antigenic epitope, βGal(1-6), with pectin, which is in many plant foods and is widely consumed. Immune response to 10A is comparable to that seen with other capsule types, and pectin ingestion may have little impact on vaccine responses. However, antibody to pectin can kill serotype 10A pneumococci and this shared epitope may be considered in pneumococcal vaccine designs.
Keywords: Streptococcus pneumoniae; bacteria; capsule; food; polysaccharide; vaccine.
Copyright © 2020 Nahm et al.
Figures


References
-
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, Patton M, McDonough A, Moradoghli-Haftvani A, Smith H, Mellelieu T, Pride MW, Crowther G, Schmoele-Thoma B, Scott DA, Jansen KU, Lobatto R, Oosterman B, Visser N, Caspers E, Smorenburg A, Emini EA, Gruber WC, Grobbee DE. 2015. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 372:1114–1125. doi: 10.1056/NEJMoa1408544. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
